COVID-19 / SARS-CoV-2
As of the 11th March 2020, the COVID-19 outbreak became a pandemic.
COVID-19 is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 was first detected in the city of Wuhan, China, in December 2019, after a cluster of patients with pneumonia of unknown cause were reported to the World Health Organization (WHO). These resources will be updated by UAR. For more resources we recommend:
- Americans for Medical Progress' COVID-19 resources
- The European Animal Research Association's interactive COVID-19 research map
- The Foundation for Biomedical Research's COVID-19 animal research round up
- The London School of Hygiene and Tropical Medicine's COVID-19 vaccine development pipeline
- Nature's graphical vaccine guide
- Coronavirus: the science explained: https://coronavirusexplained.ukri.org/en/
- World Federation of Science Journalists: https://wfsj-briefing.org
- UK scientists response: https://www.sciencemediacentre.org/tag/covid-19/
Coronaviruses (CoV) are a large family of viruses that cause illnesses that range from the common cold or mild respiratory pathologies to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). They are named Corona, because it means crown and this refers to the way the viruses look like under the microscope. Coronavirus disease (COVID-19) is a new strain that was discovered in 2019 and had not been previously identified in humans.
Coronaviruses also affect animals and they are zoonotic, meaning they can be transmitted between animals and people. Detailed investigations found that SARS-CoV was transmitted from bats to civet cats to humans and MERS-CoV from dromedary camels to humans. Several known coronaviruses are circulating in animals that have not yet infected humans.
Common signs of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death.
Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
In the rush to find vaccines and treatments, researchers have started working on the virus. Here is a list of the ongoing research involving animals:
ANIMAL MODELS
VACCINES
DRUGS
NEUTRALISING ANTIBODIES
IMMUNITY
CORONAVIRUSES IN ANIMALS
UAR Coverage
Click here for animal research against COVID-19 in the news
REFERENCES
https://www.who.int/health-topics/coronavirus
https://www.cdc.gov/coronavirus/2019-ncov/about/index.html
https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus
http://www.ox.ac.uk/news-and-events/coronavirus-research
https://www.amprogress.org/covid-19-resources/
ANIMAL MODELS :
No single kind of animal will serve all test purposes and scientists have several criteria for what makes an animal useful in testing therapies and vaccines for effectiveness. First, it must be susceptible to infection, and not all animals are. And even when laboratory animals are susceptible to infection, that doesn’t mean they get sick. If an animal doesn’t get sick from the infection, its use is limited, because testing treatment effectiveness requires observing whether the treatment stops the symptoms.
The best laboratory animal would not only get infected and get sick, but get sick in the same way that humans do, showing a similar course of disease. Then a test would give the most information.
https://www.nytimes.com/2020/03/14/science/animals-coronavirus-vaccine.html
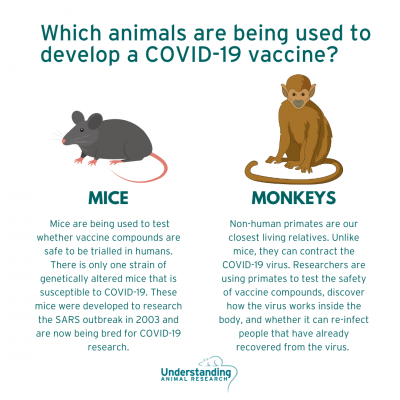
However, standard inbred strains of mice are not susceptible to COVID-19 infection. Around the world, different laboratories are racing to breed stocks of mice genetically engineered for research and testing the susceptibility of other animals to infection with the virus that causes Covid-19.
The University of Iowa developed a mouse in 2003 that was susceptible to infection with the SARS virus. It is called the hACE2 mouse. A new study from China, not yet peer-reviewed, suggests that these mice do get infected with the new pandemic virus, which is called SARS-CoV-2, and develop mild pneumonia. The advantage of this strain is that it has a human receptor on its cells called an ACE2 receptor, thus its name. That allows it to be infected with SARS and the new coronavirus, which both target that receptor as they try to invade cells.
This strain of mice will be used in some of the first laboratory experiments. But first it is necessary to breed them.
https://www.jax.org/news-and-insights/2020/march/expediting-covid-19-research
FERRETS
Ferrets can be chosen as test animals because they have the right receptor cells in its lungs to allow infection, and have proven to be a suitable animal model in the past for research into SARS, influenza and even ebola.
Researchers have determined, through genome analysis, that ferrets are the best species for creating an animal model, where vaccinated animals are protected and non-vaccinated animals come down with the disease.
https://indaily.com.au/news/2020/04/02/csiro-tests-potential-covid-19-vaccines-on-ferrets/
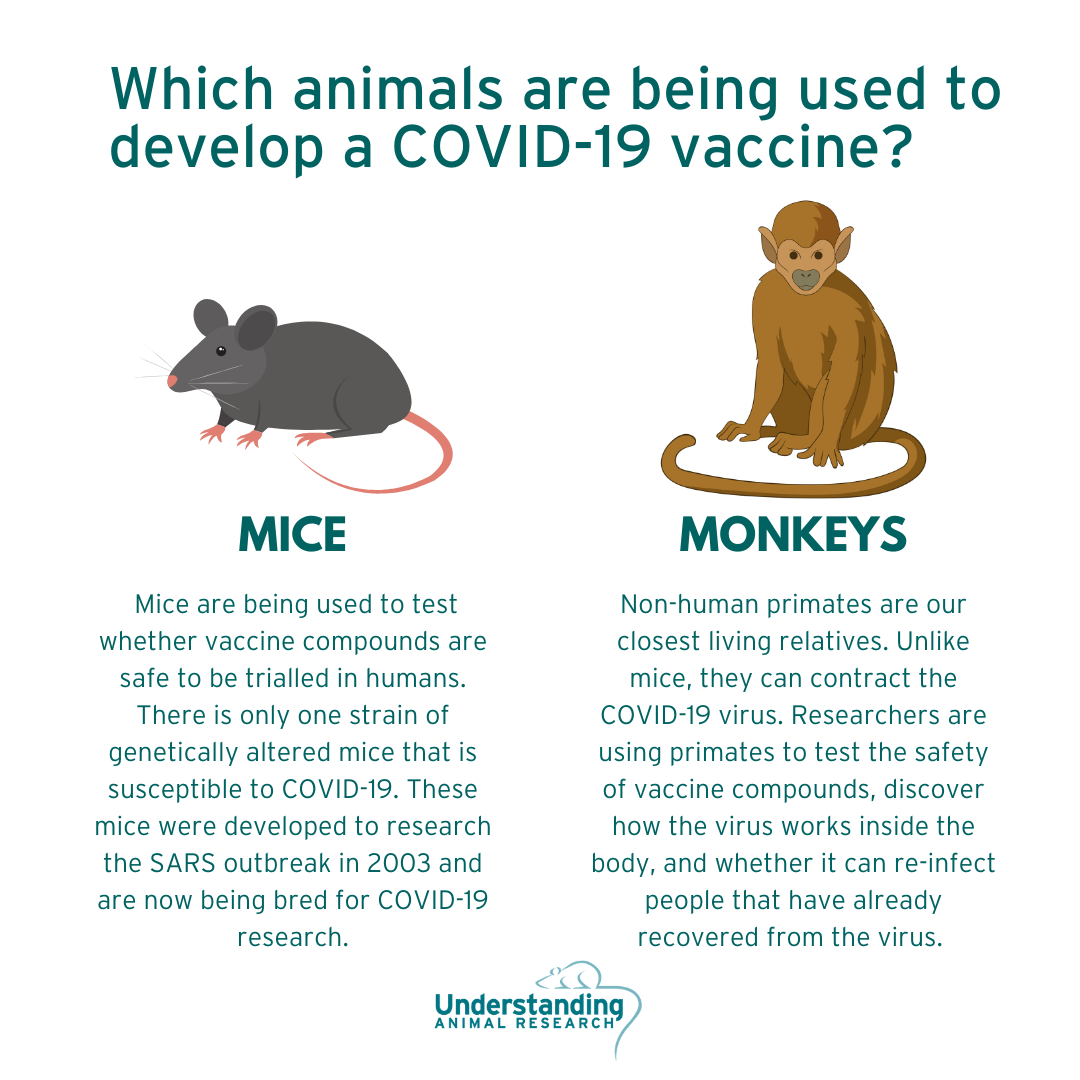
NON-HUMAN PRIMATES
Dave O’Connor, a pathologist at the University of Wisconsin, Madison, is working with colleagues to test the usefulness of monkeys in the study of coronavirus treatments. He said that a Chinese group had already published some data on rhesus macaques and he had heard that more results from other labs around the world would be coming soon.
HAMSTERS
Hamsters have an ACE2 receptor more similar to the human's compared to mice. Researchers showed 15 years ago that the Syrian hamster can be easily infected with SARS-CoV-1. At the time, the symptoms of these rodents were too subtle to arouse genuine interest in this model. But with COVID-19, the outlook is more promising.
After infection, hamsters appear to lose weight, become lethargic, their fur is tousled, their posture is hunched and they develop rapid, jerky breathing. SARS-CoV-2 is found in large quantities in the lungs and intestines of animals. And these clinical manifestations are reminiscent of upper and lower respiratory infection in humans. This model can therefore facilitate the study of the mechanics of the virus, its infection and its transmission
VACCINES :
When it comes to infectious diseases like this one, vaccines are the strongest weapons that health officials have. Getting vaccinated can protect people from getting infected in the first place.
Normally, vaccines go into human trials after tests for safety and effectiveness in animals. Researchers would take months to test for the possibility of vaccine enhancement in animals. Testing for the risk of vaccine enhancement is time-consuming because it requires scientists to breed mice that are genetically altered to respond to the virus like humans. Work on these and other animal models is just getting under way in several laboratories around the world but given the urgency to stem the spread of the new coronavirus, some drugmakers are moving straight into small-scale human tests, without waiting for the completion of such animal tests. A lot or the vaccine candidates for Covid19 are being tested in animals at the same time as human phase 1 trials are happening.
As these ‘first in human’ trials get going, key questions about how our immune system fights off the virus — and how to safely trigger a similar immune response with a vaccine — remain unanswered. Answers might come soon from studies of infected people and animal models, but some researchers say that the lack of information should not keep experts from beginning safety trials in people. Others worry that if vaccine candidates released on an accelerated schedule turn out to be ineffective or unsafe, it could send researchers back to zero and end up delaying the development and wide-scale roll-out of an effective vaccine.
However, coronavirus vaccine developers are still required to conduct routine animal testing to make sure the vaccine itself is not toxic and is likely to help the immune system respond to the virus. Researchers expect that they will learn more about the infection from both human and animal studies, and get a better sense of which vaccines are likely to work best.
About 35 companies and academic institutions are racing to create such a vaccine.
https://www.nature.com/articles/d41586-020-00798-8
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/
Vaccine development at imperial College London
Led by Professor Robin Shattock, from the Department of Infectious Disease, the Imperial team is currently working to create a viable vaccine against the novel coronavirus. They have developed a new technology that could potentially produce vaccines much faster than conventional methods. They have already successfully generated a novel SARS-Cov-2 vaccine candidate, 14 days only after receiving the genetic sequence of the virus. Animal experiments started on the 10th of February 2020. The early results in mice were encouraging. The team has now moved to testing their vaccine on monkeys with researchers in Paris. If successful, human trials could begin in early Summer. However, it would probably be a year before it was available for patients.
https://www.imperial.ac.uk/news/195055/imperial-researchers-race-develop-coronavirus-vaccine/
http://wwwf.imperial.ac.uk/blog/photography/2020/03/13/developing-the-coronavirus-vaccine/
https://www.standard.co.uk/news/health/coronavirus-covid19-vaccine-testing-a4387271.html
Vaccine development by Novavax
Novavax, Inc., a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, announced that the Coalition for Epidemic Preparedness Innovations (CEPI) awarded an initial funding of $4 million to support Novavax’ efforts to develop a COVID-19 vaccine. Novavax developed a novel MERS coronavirus vaccine candidate in 2013 which is a crucial target for coronavirus vaccine development
Novavax, which has robust vaccine technology platforms, has produced and is currently assessing multiple recombinant nanoparticle vaccine candidates in animal models prior to advancing to clinical trials. Initiation of Phase1 clinical testing is expected in late spring of 2020. Novavax’ COVID-19 vaccine candidates were created with its proprietary recombinant protein nanoparticle technology platform to generate antigens derived from the coronavirus spike (S) protein. Novavax also expects to utilize its proprietary Matrix-M™ adjuvant with its COVID-19 vaccine candidates to enhance immune responses.
mRNA-1273 Vaccine development by Moderna
Biotechnology company Moderna Inc and the Vaccine Research Center, a unit of the NIAID, have collaborated to develop a vaccine for coronavirus. The vaccine targets the Spike (S) protein of the coronavirus.
The first vials of the vaccine have been manufactured at Moderna’s Massachusetts manufacturing plant and shipped to NIAID for phase one human clinical trial. The trial began on 16 March at the Kaiser Permanente Washington Health Research Institute in Seattle, Washington. The trial is expected to take 14 months.
Testing in animals for the specific risk of vaccine enhancement, which should establish whether the vaccine is safe to expose larger numbers of people, will proceed simultaneously with human trials. And NIH will move to larger human studies only once human and animal studies confirm that the vaccine is safe. Trials that show whether a vaccine can prevent infections in people won’t proceed without such data from animals.
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/
INO-4800 Vaccine development by Inovio Pharmaceuticals
Inovio Pharmaceuticals has collaborated with Beijing Advaccine Biotechnology Company to advance the development of the former vaccine, INO-4800, as a novel coronavirus vaccine. Inovio announced an accelerated timeline for the development of the vaccine on 03 March. The company has started pre-clinical testing for clinical product manufacturing.
Researchers reported injecting Inovio’s vaccine — a DNA molecule carrying instructions to make the spike protein — into mice and guinea pigs. They found that the animals produced both antibodies and T cells against the virus. Study leader Kate Broderick, Inovio’s senior vice-president for preclinical research and development, says that her team has now given the vaccine to monkeys and is soon to start studies in which vaccinated animals are infected with the virus to see whether they are protected.
Human clinical trials in 30 healthy volunteers are expected to commence in April 2020 in the US, followed by China, and South Korea. A phase one clinical trial is planned to be conducted in parallel in China, by Beijing Advaccine. Results from the clinical trials are expected to be available in September 2020.
https://www.researchsquare.com/article/rs-16261/v1
Vaccine materials & development – University of Oxford
Oxford University will use CEPI funds to support the manufacture of vaccine materials required for animal testing and early-stage human safety trials. A research team at Oxford University’s Jenner Institute is already preparing to begin clinical testing of a novel coronavirus vaccine candidate.
The vaccines are produced using a safe version of an adenovirus; another virus that can cause a common cold-like illness. A chimpanzee adenovirus vaccine vector (ChAdOx1), developed at Oxford’s Jenner Institute, was chosen as the most suitable vaccine technology for a SARS-CoV-2 vaccine as it can generate a strong immune response from one dose and it is not a replicating virus, so it cannot cause an ongoing infection in the vaccinated individual. The adenovirus has been modified so that it cannot reproduce within the body, and the genetic code to provide instructions for making the coronavirus Spike protein has been added, enabling the adenovirus to produce this protein after vaccination. That results in the formation of antibodies to the Spike protein, which is found on the surface of coronaviruses. In someone who has been vaccinated, antibodies to the Spike can bind to the coronavirus and stop it from causing an infection.
The vaccine has started animal trials at the Public Health England (PHE) laboratory at Porton Down near Salisbury. Normally, animal work must be completed before human trials can start, but because similar vaccines have worked safely in trials for other diseases, the work has been accelerated and is happening in parallel.
https://www.ovg.ox.ac.uk/news/covid-19-vaccine-development
http://www.ox.ac.uk/news/2020-02-07-oxford-team-begin-novel-coronavirus-vaccine-research
Non-replicating viral vector by Johnson & Johnson
J&J said Feb. 11 it is expanding collaboration with Biomedical Advanced Research and Development Authority, or BARDA, with the latter providing funding for accelerated development of the vaccine candidate.
J&J has the experience of working on similar vaccine candidates for viral threats such as Zika and Ebola. The company said last week that it has entered into a collaboration with the Beth Israel Deaconess Medical Center to support development of a vaccine for COVID-19. The company and hospital have embarked on preclinical testing of multiple vaccine prospects, targeting the shortlisting of one by the end of March and initiation of a Phase 1 clinical study of by the end of 2020. They already have a lot of data on the safety of the vaccine candidate, because the necessary preclinical studies in non-human primates and animals have already been carried out. Thanks to the close cooperation with the authorities, they can now move much faster.
J&J said it is developing animal models to test for vaccine enhancement and hopes to have a vaccine candidate ready for human trials in October. A Sanofi spokeswoman said the company will examine this risk before testing the vaccine in clinical trials. researchers are already “challenging” vaccinated animals with SARS-CoV-2 to see which responses best correlate with protection.
https://finance.yahoo.com/news/8-biotechs-coronavirus-vaccines-development-144215778.html
Sinovac Biotech's SARS strategy vaccine
Sinovac Biotech is making a SARS-CoV-2 vaccine by chemically inactivating whole virus particles and adding an immune booster called alum. Sinovac used the same strategy for a SARS vaccine it developed and tested in a phase I clinical trial 16 years ago, says Meng Weining, a vice president at Sinovac. “We immediately just restarted the approach we already know.” The company’s SARS vaccine worked in monkeys and although there were concerns that an inactivated coronavirus vaccine might trigger the sort of antibody enhancement disease that occurred with the RSV vaccine, Meng stresses that no such problems surfaced in their animal studies.
A nonreplicating version of adenovirus-5 (Ad5) vaccine by CanSino
CanSino is now testing a vaccine that uses a nonreplicating version of adenovirus-5 (Ad5), which also causes the common cold, as a “vector” to carry in the gene for the coronavirus spike protein. Other vaccine researchers worry that because many people have immunity to Ad5, they could mount an immune response against the vector, preventing it from delivering the spike protein gene into human cells—or it might even cause harm, as seemed to happen in a trial of an Ad5-based HIV vaccine made by Merck that was stopped early in 2007. But the same Chinese collaboration produced an Ebola vaccine, which Chinese regulators approved in 2017, and a company press release claimed its new candidate generated “strong immune responses in animal models” and has “a good safety profile.”
Altimmune’s intranasal coronavirus vaccine
An intranasal Covid-19 vaccine is being developed by US-based clinical-stage biopharmaceutical company, Altimmune. Design and synthesis of the single-dose vaccine have been completed, while animal testing will follow.
The coronavirus vaccine is being developed based on a vaccine technology platform that is similar to NasoVAX, an influenza vaccine developed by Altimmune.
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/
Avian Coronavirus Infectious Bronchitis Virus (IBV) vaccine by MIGAL Research Institute
The MIGAL Research Institute in Israel announced that an Infectious Bronchitis Virus (IBV) vaccine developed to treat avian coronavirus has been modified to treat COVID-19. The vaccine has demonstrated efficacy in pre-clinical trials conducted by the Volcani Institute.
The IBV vaccine was developed after four years of research and has high genetic similarity to the human coronavirus. The institute has genetically modified the vaccine to treat COVID-19 and will be available in the oral form.
The institute is currently exploring potential partners for producing the vaccine in the next eight to ten weeks and obtaining the necessary safety approvals for animal testing.
https://www.israeltoday.co.il/read/israeli-breakthrough-for-coronavirus-vaccine-imminent/
TNX-1800 by Tonix Pharmaceuticals
Tonix Pharmaceuticals has partnered with Southern Research, a non-profit research organisation, to develop a coronavirus vaccine named TNX-1800. The vaccine is a modified horsepox virus developed using Tonix’s proprietary horsepox vaccine platform.
TNX-1800 is designed to express a protein derived from the virus that causes the coronavirus infection. Southern Research will be responsible for evaluating the efficacy of the vaccine, under the partnership.
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/
Recombinant subunit vaccine by Clover Biopharmaceuticals
Clover Biopharmaceuticals is developing a recombinant subunit vaccine using its patented Trimer-Tag©technology. The company is developing the vaccine based on the trimeric S protein (S-Trimer) of the COVID-19 coronavirus, which is responsible for binding with the host cell and causing a viral infection.
Using Trimer-Tag© technology, Clover successfully produced the subunit vaccine in a mammalian cell-culture based expression system on 10 February. The company also identified antigen-specific antibody in the serum of fully recovered patients who were previously infected by the virus. A highly purified form of the S-Trimer vaccine is expected to be available in six to eight weeks for performing pre-clinical studies. The company is equipped with in-house cGMP biomanufacturing capabilities to scale-up production if the vaccine is proven to be successful. Clover is also collaborating with GSK to develop a vaccine using the latter’s pandemic adjuvant system.
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/
Linear DNA Vaccine by Applied DNA Sciences and Takis Biotech
Applied DNA Sciences’ subsidiary LineaRx and Takis Biotech formed a joint venture on 07 February to develop a linear DNA vaccine as a treatment for coronavirus. They will use Polymerase Chain Reaction (PCR)-based DNA manufacturing technology to develop the vaccine. It offers several advantages including high purity, increased production speed, and absence of antibiotics and bacterial contaminants. Further, the vaccine gene developed through this technology can be effective without being inserted into the patient’s genome.
The design for four DNA vaccine candidates is expected to be produced using the PCR technology for carrying out animal testing. The design of one of the vaccine candidates is based on the entire spike gene of the coronavirus, while the remaining are designed based on the antigenic portions of the protein. Takis will likely begin animal testing in the second quarter of 2020.
https://finance.yahoo.com/news/8-biotechs-coronavirus-vaccines-development-144215778.html
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/
Pirbright to test new coronavirus vaccines on animals
Working in collaboration with researchers at the University of Oxford and Public Health England, a team of scientists at Pirbright will begin testing new vaccines for their ability to induce protective antibodies against SARS-CoV-2. The vaccines will include the chimpanzee adenovirus vaccine vector (ChAdOx1) which is soon to enter human phase I clinical trials and has been used to create vaccines for diseases like Ebola, Middle Eastern respiratory syndrome (MERS) and flu.
The vaccine candidates developed at Oxford will contain the spike protein from SARS-CoV-2, the protein against which protective antibodies are generated in infected patients. Pirbright scientists will measure the level of antibodies produced after vaccination of pigs and assess whether the antibodies can block SARS-CoV-2 from infecting cells, thereby preventing infection. Importantly, the pig immune system shares significant similarities to that of humans, so a good response to a vaccine in pigs will help to predict the success of vaccines for human use. Researchers will also test the safety of the new vaccines and monitor whether any adverse effects are observed in the pigs.
Vaccine by the University of Pittsburgh School of Medicine tested in mice
University of Pittsburgh School of Medicine scientists today announced a potential vaccine against SARS-CoV-2, the new coronavirus causing the COVID-19 pandemic. When tested in mice, the vaccine, delivered through a fingertip-sized patch, produces antibodies specific to SARS-CoV-2 at quantities thought to be sufficient for neutralizing the virus.
SARS vaccine in ferrets
Severe acute respiratory syndrome (SARS) caused by a the coronavirus SARS-CoV emerged in humans in 2004. In the search for a vaccine, researchers immunized ferrets with recombinant modified vaccinia virus Ankara (rMVA) expressing the SARS-CoV spike (S) protein. Immunized ferrets developed a more rapid and vigorous neutralizing antibody response than control animals after challenge with SARS-CoV; however, they also exhibited strong inflammatory responses in liver tissue. Inflammation in control animals exposed to SARS-CoV was relatively mild. The data suggested that vaccination with rMVA expressing SARS-CoV S protein is associated with enhanced hepatitis.
https://jvi.asm.org/content/78/22/12672
DRUGS :
Remdesivir - University of Nebraska Medical Center - NIH
A randomized, controlled clinical trial to evaluate the safety and efficacy of the antiviral remdesivir in hospitalized adults diagnosed with COVID-19 has begun at the University of Nebraska Medical Center (UNMC) in Omaha. Remdesivir had been developed for Ebola. Remdesivir showed encouraging results among animals infected with two related coronaviruses, responsible for SARS and MERS. Volunteers will be randomly assigned to receive either the drug or a placebo intravenously for 10 days, and they will have blood tests and nose and throat swabs taken every two days to track the amount of virus in their bodies. Even if the drug shows some efficacy in keeping blood levels of SARS-CoV-2 from growing, it could help to contain spread of the infection.
https://time.com/5790545/first-covid-19-vaccine/
https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins
NP-120 (Ifenprodil) by Algernon Pharmaceuticals
Algernon Pharmaceuticals has announced that it is exploring its NP-120 (Ifenprodil) as a potential treatment COVID-19. Ifenprodil is an N-methyl-d-aspartate (NDMA) receptor glutamate receptor antagonist sold under the brand name Cerocal. It has demonstrated efficacy in improving survivability in mice infected with H5N1. Ifenprodil showed a 40% improvement in mortality and significantly reduced acute lung injury (ALI) in a recent H5N1 animal study.
“If we see a similar response in coronavirus patients as seen in the H5N1 animal study, a far more lethal form of influenza, we believe that patient outcomes should be significantly improved”, said Christopher J. Moreau, CEO of Algernon.
Niclosamide and ciclesonide - Pasteur Institute in Korea
Drug repositioning represents the only feasible option to address this global challenge and a panel of 48 FDA-approved drugs that have been pre-selected by an assay of SARS-CoV was screened to identify potential antiviral drug candidates against SARS-CoV-2 infection.
Researchers found a total of 24 drugs which exhibited antiviral efficacy (0.1 μM < IC50 < 10 μM) against SARS-CoV-2. In particular, two FDA-approved drugs – niclosamide and ciclesonide – were notable in some respects. These drugs will be tested in an appropriate animal model for their antiviral activities. In near future, these already FDA-approved drugs could be further developed following clinical trials in order to provide additional therapeutic options for patients with COVID-19.
https://www.biorxiv.org/content/10.1101/2020.03.20.999730v3
EIDD-2801 - University of North Carolina
Scientists are hopeful that a new drug, called EIDD-2801, could change the way doctors treat COVID-19. The drug shows promise in reducing lung damage, has finished testing in mice and will soon move to human clinical trials. The study found that, when used as a prophylactic, EIDD-2801 can prevent severe lung injury in infected mice. EIDD-2801 is an orally available form of the antiviral compound EIDD-1931; it can be taken as a pill and can be properly absorbed to travel to the lungs.
When given as a treatment 12 or 24 hours after infection has begun, EIDD-2801 can reduce the degree of lung damage and weight loss in mice. This window of opportunity is expected to be longer in humans, because the period between coronavirus disease onset and death is generally extended in humans compared to mice.
NEUTRALISING ANTIBODIES
Human antibody to new coronavirus works in mice – Utrecht University
Monoclonal antibodies targeting vulnerable sites on viral surface proteins are increasingly recognised as a promising class of drugs against infectious diseases and have shown therapeutic efficacy for a number of viruses. Coronavirus neutralizing antibodies primarily target the trimeric spike (S) glycoproteins on the viral surface that mediate entry into host cells.
In order to identify SARS-CoV-2 neutralizing antibodies, ELISA-(cross) reactivity was assessed of antibody-containing supernatants of a collection of 51 SARS-S hybridoma’s derived from immunized transgenic H2L2 mice that encode chimeric immunoglobulins with human variable heavy and light chains and constant regions of rat origin. This cross-neutralizing antibody targets a communal epitope on these viruses and offers potential for prevention and treatment of COVID-19.
https://www.uu.nl/en/news/human-antibody-to-new-coronavirus?mc_cid=b47e37d54a&mc_eid=ec597d271e
https://www.biorxiv.org/content/10.1101/2020.03.11.987958v1.full
Antibody that neutralises Covid-19 – VIB-UGent
The lab of Xavier Saelens (VIB-UGent) announced the discovery of a unique antibody that is capable of binding the virus that causes COVID-19 (SARS-CoV-2) and neutralizing it. The antibody could prevent the new coronavirus from infecting human cells. Importantly, the antibody can also be produced at large-scale using production processes that are common in the biopharmaceutical industry.
In contrast to vaccines, an antibody offers immediate protection – though of shorter duration. The advantage of this approach over vaccines is that patients don’t need to produce their own antibodies. The most vulnerable groups, such as the elderly, often mount a modest response to vaccines, which means that their protection may be incomplete. Healthcare workers or people at increased risk of exposure to the virus can also benefit from an immediate protection. This type of medicine can therefore be an important tool in fighting the current pandemic.
TZLS-501 by Tiziana Life Sciences
Tiziana Life Sciences is developing its monoclonal antibody named TZLS-501 for the treatment of COVID-19. TZLS-501 is a human anti-interleukin-6 receptor (IL-6R), which helps in preventing lung damage and elevated levels of IL-6 in vitro and in vivo.
The drug works by binding to IL-6R and depleting the amount of IL-6 circulating in the body thereby reducing chronic lung inflammation.
https://www.tizianalifesciences.com/our-drugs/milciclib/clinical-trials
Llama Antibodies
Scientists say llamas could help defeat the coronavirus.
According to a new study published in the journal Cell by an international team of researchers, antibodies found in the blood of llamas were able to stave off COVID infections.
Cow antibodies
A biotech company has coaxed genetically modified cows to pump out human antibodies that subdue SARS-CoV-2, the pathogen causing the deadly disease, and it plans to start clinical trials of them this summer.
IMMUNITY
Immunity development - Institute of Laboratory Animal Science, Beijing
A team based in China looked at two rhesus macaques (Macaca mulatta) that had recovered from SARS-CoV-2 infection, which caused them only mild illness. The monkeys did not seem to become re-infected when researchers exposed them to the virus for a second time four weeks after their initial exposure. Researchers will be looking for evidence that humans react in the same way, for instance by studying people potentially exposed multiple times.
https://www.biorxiv.org/content/10.1101/2020.03.13.990226v1.full.pdf
https://www.genengnews.com/news/covid-19-reinfection-not-a-concern-monkey-study-suggests/
https://www.the-scientist.com/news-opinion/monkeys-develop-protective-antibodies-to-sars-cov-2-67281
CORONAVIRUSES IN ANIMALS
Human coronavirus OC43 outbreak in wild chimpanzees
A number of pathogens have been described to circulate between humans and non-human primates. The close relatedness between these hosts is thought to support pathogen transmission. Due to their rapid spread and difficult containment, airborne pathogens raise the greatest concerns. Common human respiratory viruses such as the human respiratory syncytial virus (HRSV), the human metapneumovirus (HMPV) and the human rhinovirus C, have caused lethal outbreaks in wild habituated great apes. Researchers report the transmission of the human coronavirus (HCoV) OC43 to wild chimpanzees (Pan troglodytes verus) living in the Taï National Park, Côte d´Ivoire. Between late December 2016 and early January 2017, a mild respiratory outbreak was observed in the East chimpanzee community.
https://www.tandfonline.com/doi/full/10.1038/s41426-018-0121-2?scroll=top&needAccess=true
Infection with SARS-CoV-2 Causes Pneumonia in the Rhesus Macaques
Coronavirus infections are common in humans and other mammals. Animal models are essential for the study of pathogenesis of the viral infection, the evaluation of potential antiviral treatments or vaccine development. Researchers report a nonhuman primate disease model for SARS-CoV–2. They experimentally infected Rhesus macaques using SARS-CoV–2 isolation from clinical bronchoalveolar lavage fluid and evaluated the dynamics of SARS-CoV–2 in the blood, swabs and respiratory tract tissues of Rhesus macaques.
https://www.researchsquare.com/article/rs-15756/v1
Cats and ferrets can get the new coronavirus, but no evidence that they spread it to humans
Cats can be infected with the new coronavirus, SARS-CoV-2, and can pass it on to other cats, according to a non–peer-reviewed study published on the bioRxiv preprint server yesterday. But experts caution the work was done on a small number of lab animals, which were infected with high doses of the virus. None of the cats showed COVID-19 symptoms, and there is no evidence that felines could infect people with the virus, Nature reports. Research during the 2003 outbreak of severe acute respiratory syndrome (a coronavirus relative of SARS-CoV-2), showed that cats could become infected with that virus, but they did not appear to play a role in spreading the disease. The U.S. Centers for Disease Control and Prevention says right now there is no evidence that cats or other pets can spread COVID-19.
Reinfection could not occur in SARS-CoV-2 infected rhesus macaques
It remains unclear whether the convalescing patients have a risk of “relapse” or “reinfection”. The longitudinal tracking of re-exposure after the disappeared symptoms of the SARS-CoV-2-infected monkeys was performed. Researchers found that weight loss in some monkeys, viral replication mainly in nose, pharynx, lung and gut, as well as moderate interstitial pneumonia at 7 days post-infection were clearly observed in rhesus monkeys after the primary infection. After the symptoms were alleviated and the specific antibody tested positively, the half of infected monkeys were rechallenged with the same dose of SARS-CoV-2 strain. Notably, neither viral loads in nasopharyngeal and anal swabs along timeline nor viral replication in all primary tissue compartments at 5 days post-reinfection was found in re-exposed monkeys. Combined with the follow-up virologic, radiological and pathological findings, the monkeys with re-exposure showed no recurrence of COVID-19, similarly to the infected monkey without rechallenge. Taken together, the results indicate that the primary SARS-CoV-2 infection could protect from subsequent exposures, which have the reference of prognosis of the disease and vital implications for vaccine design.
https://www.biorxiv.org/content/10.1101/2020.03.13.990226v1
Considerations for Great Apes in human care
A number of pathogens have been described to circulate between humans and non-human primates. The close relatedness between these hosts is thought to support pathogen transmission. The corona viruses responsible for SARS, MERS and now SARS-CoV-2 (the corona virus that causes COVID-19) have dramatically altered the pathogenicity and threat to human populations of this family of coronaviruses. Currently- there is no data supporting host-specific CoVs in great apes and other primates, but there is a published report on anthroponotic transmission of HCoV-OC43 (the corona virus that causes the “common cold” in humans) resulting in respiratory disease in chimpanzees. It is important to remember that there have been no studies looking at the transmission potential or pathogenicity of SARS-CoV-2 between humans and great apes. Despite this, it is the feeling of the great ape veterinary advisors that the emergence of this novel corona virus may pose a significant health risk to great apes in human care.
https://zahp.aza.org/wp-content/uploads/2020/03/COVID-19-and-Great-Apes_3.12.2020.pdf
UAR Coverage
- Warning signs of the coronavirus: why we knew about it and couldn't stop it
- Can your cat catch coronavirus?
- Is COVID-19 isolation making your dog as anxious as you?
- Can we fight COVID-19 without animal testing?
- High public acceptance of animal research to find treatments for COVID-19
- Could llama antibodies treat viruses like COVID-19?
- Remdesivir trials effective against coronaviruses in animals and humans
- What mice studies can tell us about the effect of anxiety on sleep
- Masks reduce COVID-19 transmission between hamsters
- COVID-19 research exposes activists' lack of evidence
- How organoids are bringing the study of bats to the lab
Last edited: 15 June 2020 21:31