Brain tumours
A brain tumour is a collection of brain cells that have grown out of control. A tumour can originate from any part of the brain.
The brain is made of a lot of different tissues and cells which can each develop into different types of tumours. There are over 120 different types of brain tumours. Tumours are usually named after the type of cell they develop from. The most common type of brain tumours in adults are called gliomas and originate from glial cells.
Different brain tumours
Not all brain tumours are brain cancers, but even benign (or noncancerous) tumours can be dangerous because of their size or location.
Tumours that start in the brain are called primary brain tumours. They are relatively rare. Around 12,300 people are diagnosed with a brain tumour in the UK each year. Brain tumours are more common in older people. Almost 25% of patients with a brain or spinal cord tumour in the UK are aged 75 or older. It is important to note, however, that tumours affecting the brain and spinal cord are the second most common type of children’s cancer in the UK.
This is different from cancers that have spread to the brain from somewhere else in the body. These are called secondary brain cancers or brain metastases. The secondary cancer is made of the same type of cells as the primary cancer. So, if the cancer started in the lung and spread to the brain, the areas of cancer in the brain are made up of lung cancer cells. Any cancer can spread to the brain. The most common cancers that do are lung cancers, breast cancers, kidney cancers, melanoma skin cancers, and bowel cancers (colorectal cancers).
Brain tumour symptoms depend on where the cancer is in the brain. The cancer can cause pressure on the surrounding brain tissue and the symptoms will depend on what this part of the brain does.
Brain tumours are not always cancerous. They can be benign (non cancerous) or malignant (cancerous).Benign tumours are usually low grade and grow slowly. They are less likely to come back after treatment or to spread to other parts of the brain. Each year around 6,500 people are diagnosed with a benign or an uncertain or unknown behaviour brain tumour in the UK. Malignant brain tumours are high grade and grow faster than benign tumours. They are more likely to come back after treatment and to spread to other parts of the brain. Each year around 5,800 people are diagnosed with a malignant brain tumour in the UK.
Treatment
Treatment for a brain tumour aims to remove as much of it as possible and try to stop it coming back. The main treatments are:
- Surgery – a small section of skull is removed and the tumour is cut out before the piece of skull is fixed back in place. The development of various animal models has refined brain surgery techniques, allowing for successful tumour resections.
- Radiotherapy – radiation from an external machine is used to kill cancer cells. Radiotherapy is a central element of multimodal cancer treatment. It has experienced tremendous advancements in the recent years and decades that have turned it into a versatile tool of high precision radiosurgery. These improvements, including the implementation of new imaging modalities, the improvement of treatment planning algorithms, the development of image-guidance techniques for dose administration, and the advent of new irradiation qualities, have all been evaluated through careful and thorough preclinical testing in appropriate animal models.
- Radiosurgery – lots of tiny beams of radiation are aimed at the cancer to kill it if you can't have surgery
- Chemotherapy – medicine is used to kill cancer cells after surgery, or relieve symptoms if the tumour can't be removed. Animal models have been and are still currently used to assess the efficacy of these chemo-preventive agents. Observations made in these models are often used to determine which drugs are qualified to progress to clinical trials. Organ specific animal models are employed to determine which agents or classes of agents are likely to be the most effective to prevent organ-specific forms of cancer.
- Carmustine implants (glial wafers) – a new way of giving chemotherapy for some high-grade tumours, where implants are inserted into the brain. Studies in animals have notably demonstrated the capability of this system to produce targeted high dose-delivery within millimetres of the implant.
- Immunotherapy – uses substances made by the body or in a laboratory to boost the immune system and help the body find and destroy cancer cells. Immunotherapy has revolutionised cancer therapy generally, but is not yet effective in brain cancers. A major part of the success of immunotherapy has been the development of appropriate mouse models. Faithful mouse models that recapitulate the complexity of human malignancy and immune contexture within the tumour microenvironment are crucial to interrogate and predict antitumour immune responses and therapeutic efficacy in clinical trials.
Medicines may also be used to relieve symptoms like headaches, seizures and being sick (vomiting).
Animal models of primary brain tumours
There are two main reasons for modelling brain tumours in animals. The first is to identify the genetic events and molecular mechanisms that contribute to oncogenesis within the central nervous system, and the second is to evaluate potential therapeutic strategies. For both applications, in vitro models - alone - are deemed insufficient.
Cell lines derived from brain tumours are useful for the characterisation of the genetic lesions that occur in human tumours, and to build primary hypothesis about gene function. However, they cannot model effectively the key aspects of tumourigenesis, such as microenvironment contribution, invasion, angiogenesis or inflammatory response. Therefore, they only have a limited predictive value for the understanding of the disease and the development of cancer therapies. In vivo models provide a more accurate experimental system, as they mimic tumour behaviour in an entire mammalian organism.
The goal of a model is to reproduce the aetiology and biology of the corresponding human cancer. As such, a model should ideally display the same genetic lesions, in the same anatomical location, with corresponding histopathological features, and in the same developmental time frame as the human tumour. It is also important that the modelled tumour recapitulate intertumoral and intra-tumoral heterogeneity and be predictive of the patients’ response to treatment. This has not always been easy to do. Researchers have developed different animal models across the years aiming for a gold standard.
Numerous animal models have been developed over the past 60 years to study brain tumour initiation, progression and development. The first came from patient material transplanted into immunocompetent rodents (xenografts) in the 1940s and 1950s.
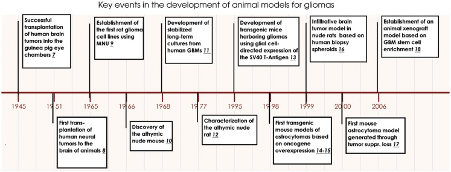
Key events in brain tumor modeling in animals. Milestones in brain tumor model development starting with transplantation of human xenografts into immunocompetent rodents in the 1940s through rodent carcinogenesis and human monolayer cell line development in the 1960s, toward the establishment of GEM models in the 1990s, and finally the establishment of xenograft models based on stem cell enrichment.
Today, the animal models of primary brain tumours can be divided into 3 categories:
- chemically induced models
- xenographs
- genetically modified mice
Chemically induced animal models of brain tumours
Among the chemically induced models, the rat has been one of the most widely used. Since the mid-1970s several rat brain tumour models have been developed. Gliomas can be induced in rats injected with cancer causing agents, notably alkylating agents (N-methylnitrosourea or N-ethyl-N-nitrosourea). These rats models have the advantage of spontaneously developing de novo tumours, preserving tumour–host interactions.
Spontaneous brain tumours are rarely reported in animals, except in dogs, where the incidence and malignancy types are similar to those seen in humans. Murine, canine, and feline models also exist but have gained less popularity.
About 1% of the rat strains that are commonly used for research purposes develop brain tumours spontaneously. Experimental tumours can also be induced by local, oral, intravenous, or transplacental exposure of cancer-causing compounds into adult or pregnant rats.
It is important to note that in these chemically brain tumours appear to be quite different from human gliomas and have frequently been referred to as “gliosarcomas” or “glioma-like tumours”. They grow as circumscribed tumours, instead of being invasive like the human tumours. Moreover, chemical induction of brain carcinogenesis seems to vary from one species to the next. It has notably been less successful in mice and no single chemical agent has yet (2010) been implicated in human brain tumour development. This doesn’t mean that there are none, and is most likely linked to deliberate exposure to toxic doses of a drug is impossible in humans, who are often in contact with the same drugs but sporadically at trace levels. This has hampered their use as rat tumours are far less characterised at the molecular level and thus it is not known to what extent their mutational and transcriptional profiles match those of human tumours.
The use of syngeneic chemically induced brain cancers has been hampered by this. However, they have brought much information about the mechanisms behind chemically induced mutagenesis within the central nervous system. Improving our knowledge of the molecular, histological and genetic profiles of these induced rat tumours would however make them a powerful tool to evaluate therapeutic responses.
Xenograft models of brain tumours
Xenograft models are generated by the transplantation of biopsies or cultured cells derived from human brain tumours into immunodeficient mice. Biopsy spheroid xenograft models are based entirely on fresh brain tumour biopsies that are engrafted into immunodeficient animals. The most prominent are heterotopic-to-orthotopic xenograft models and orthotopic biopsy spheroid models.
These cells harvested from patient tumours are usually grown in serum-containing medium and are, on their own, unlimited source of material for drug testing. However, tumours initiated from cultured cells often poorly resemble the genotype and phenotype of their parental tumours. They grow as circumscribed, non-invasive tumours and do not display the same transcriptomic profile and genomic alterations as the parental tumours.
However, when transplanted in an animal, they regain some of their original functions. The establishment of tumours in animals by xenografting tumour material, has been highly valuable in the search for mechanisms that determine tumour formation, growth, and progression. The advent of immunodeficient animals, in particular, helped gain important insight relating to the growth of human tumours within the central nervous system.
Preclinical trials have been mostly performed using mouse xenografts of human brain tumour cell lines. However, fundamental differences between cell-line derived models and patients’ tumours might have hindered translation of findings into successful results in subsequent clinical trials.
Genetically engineered mouse (GEM) models of brain tumours (including models induced via DNA or RNA- containing viruses)
During the last decades, the in-depth characterisation of genomic alterations in brain tumours has led to a better understanding of the genetic mutations and alterations that underlie the initiation and progression of several primary brain tumour subtypes. With the development of advanced genomic tools, highly characterised genetically engineered mouse (GEM) models of glioma were created based on specific genetic alterations observed in human tumours.
In many instances, these GEM models reflect the histopathology, aetiology, and biology of human brain tumours. Compared to xenotransplantation models, these GEM models more faithfully reflect important characteristics of several brain tumour subtypes and have provided insights into specific genetic events that trigger tumour initiation and progression. As such, they represent an important experimental tool.
GEM models provide elaborate temporally and genetically controlled systems to investigate the cellular origins of brain tumours and gene function in tumorigenesis. Not only have GEM models provided important new insight into the molecular and cellular events and pathways responsible for tumour initiation, progression, and metastasis, but they have also supported testing of new therapeutic strategies.
Naturally, GEM models represent an excellent tool to dissect the minimum genetic alterations that are necessary for malignant transformation and to define the interplay among different pathways involved in oncogenesis. However, GEM models can also address specific molecular events that lead to cancerous growth, model tumour/stroma interactions that contribute to malignancy, as well as the relationship with the immune system that can contribute to malignant progression. As such, since GEM models have been established in immunocompetent animals, they have expanded our knowledge of the important role of the microenvironment in tumour biology. It is highly likely that comprehensive molecular and cellular characterization of GEM models will lead to the identification of biomarkers for early stages of tumour development.
However, to grasp the depth of translatable knowledge that will emanate from these GEM models, it is important to account for the differences in mouse genetic background modifiers, as well as possible differences in tumour development and drug response between mice and humans. Therefore, it is essential to integrate accurate GEM models of brain tumours with xenograft models, genomic and developmental studies, to provide powerful platforms for target identification and drug testing.
The limits of animal models of brain tumours
None of the current animal models fully reflects human brain tumours. Most if not all of the brain tumour models used today don’t recapitulate the full genomic and phenotypic signatures of human tumours. Despite these limitations, the last sixty years of model development have been instrumental for our current understanding of how gliomas develop.
These models have been far from useless. The GEM and xenograft models derived from neuro-sphere cultures and human xenograft biopsies provide excellent tools to address a very important question: What are the exact mechanisms that lead to single tumour cell infiltration into the normal brain? These models have provided important insights into specific mechanisms of tumour initiation, progression and development.
Unfortunately, the knowledge gained from these models has only to a limited extent been translated into more effective treatment principles. The predictive value of animal models for clinical therapy is often linked to the fact that:
- the tumour models do not reflect the biological properties of the patient tumours
- the animals used do not display the same pharmacokinetics as humans
- the tumours established do not reflect the cellular heterogeneity of human tumours
However, it is anticipated that a combined comprehensive knowledge of the various models currently available will provide important new knowledge on target identification and the validation and development of new therapeutic strategies, not least because significant improvements have been made recently with the establishment of highly invasive glioblastoma models.
Last edited: 29 March 2023 11:21
