Endometriosis
Endometriosis is the name given to the condition where cells similar to the ones in the lining of the womb (uterus) are found elsewhere in the body. From puberty to menopause, each month these cells react in the same way as those in the womb, building up and then breaking down and bleeding. Unlike the cells in the womb that leave the body as a period, this blood has no way to escape. In the UK, around 1.5 million women are currently living with the condition. Endometriosis can affect you from puberty to menopause, although the impact may be felt for life.
https://www.endometriosis-uk.org/what-endometriosis
Endometriosis causes a chronic inflammatory reaction that may result in the formation of scar tissue (adhesions, fibrosis) within the pelvis and other parts of the body. Endometriosis is not limited to the area immediately in and around the womb and can spread to all kinds of places, including the colon, for example.
Endometriosis can have a significant impact on a person's life in a number of ways, including:
• Chronic pain
• Fatigue/lack of energy
• Depression/isolation
• Problems with a couple’s sex life/relationships
• An inability to conceive
• Difficulty in fulfilling work and social commitments
Causes of endometriosis remain elusive
Even though endometriosis was first identified more than 100 years ago, the mechanisms underlying the development and maintenance of this condition are far from being understood. Endometriosis is a complex disease that remains quite elusive, despite the number of people that are affected globally. There are several theories about the cause of endometriosis, but none fully explains why endometriosis occurs. These are:
Lymphatic or circulatory spread
https://www.endometriosis-uk.org/causes
Additionally, the lack of reliable non-invasive methods to detect endometriosis at any stages of the disease, but especially in the early stages, has made it very difficult to study the early development of the condition and how it progresses in humans.
Observation of endometriosis relies on a surgical procedure, a laparoscopy, where a surgeon passes a thin tube through a small cut to see any patches of endometriosis tissue It is impossible to monitor the progression of this disease without repeated laparoscopies.
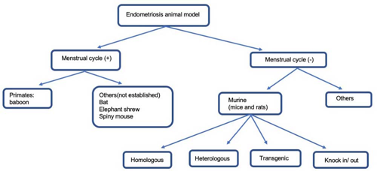
Animal models of endometriosis
A range of species have been used to study endometriosis, including rats, mice, rabbits, hamsters and non-human primates. The most important difference among these models is that rats, mice, rabbits, and hamsters do not have natural menstrual cycle and hence develop endometriosis spontaneously, whereas non-human primate do.
For a disease as diverse as endometriosis, a single animal model is unlikely to encompass the full diversity in aetiology, pathogenesis, and pathology. Researchers are more likely to find models that represent certain aspects of the disease – each model having design-related strengths and limitations that scientists need to consider to answer specific hypotheses.
The animal models used in the early stages of drug development, usually rely on non-menstruating modelswith induced endometriosis-like lesions.
For more information on the contributions of animal models to endometriosis research see: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-020-02471-0
Source:https://www.imrpress.com/journal/FBE/13/1/10.2741/871/htm
Rodent models
The rodent endometriosis models - mostly and predominantly mice but also rats - are widely used in research. They are extensively used to study the aetiology, pathology, and risk factors of endometriosis as well as to explore and evaluate novel therapeutic approaches.
Mice and rats lack a menstrual cycle and do not spontaneously develop endometriosis. Endometriosis can be induced in mice. There are several ways to imitate endometriosis:
Homologous or autologous rodent models
In these models, “healthy’” uterus tissue from animals is cut into fragments and transplanted into the peritoneum of recipient animals to mimic the pelvic environment in human endometriosis. There, it starts to grow in an oestrogen-dependent manner. These models were developed based on the implantation theory : the idea that endometriosis is caused by endometrial tissue that flows (retrogradely) through the fallopian tube and into the peritoneal cavity where it attaches to and invades tissues and organs within the cavity.
In the autologous or syngeneic rodent model, the immune system is intact, and this offers the possibility to study the cross-talk between the immune system and endometriotic cells within the peritoneal microenvironment, which was shown to play a key role in humans.
Autologous models have been established in rats, mice, hamsters and rabbits, but mainly rat and mouse models have been further developed in recent years. In particular, researchers developed a ‘green mouse model’ that ubiquitously express green fluorescent protein (GFP) as donors. Transplanting fluorescent endometrial tissue into recipient mice makes it easier to seeendometriotic lesions.
Heterologous rodent model
In heterologous models, human endometriotic lesions are transplanted into the peritoneal cavity of immune-deficient mice, and into the peritoneum of immunocompromised mice. Although these models have the great advantage of investigating human endometrial tissues in vivo, the animals cannot mimic the inflammatory response normally seen in human endometriotic lesion. The lack of immunocompetence represents a significant factor that limits the investigation of several molecular and cellular pathways.
Moreover, the number of endometriotic lesions developed in the heterologous models varies from one animal to another. Hence, although heterologous rodent endometriosis models are responsive to drugs and manipulations that induce a hypoestrogenic state, it may be difficult to analyse novel target families.
Transgenic models
In recent decades, many knockout or transgenic mice have been generated. Knockout and transgenic mouse models are helpful to study biological factors such as the role of immune system and hormone balance that may affect endometriosis. These models also enable the evaluation of molecular mechanisms that are critical for disease initiation. Currently there is an increased interest in using genome wide association studies to better understand the risks/aetiology of endometriosis. New insights into the genetics of the disease might lead to new transgenic animal models of the disease.
It is important to keep in mind that rodent models have limitations and do not mimic all aspects of human pathophysiology. Ideally, disease models should mimic human disease and allow scientific investigation of the effects of both intrinsic factors, such as genes, and extrinsic factors, such as environmental factors on disease progression. Transplanting normal endometrium into the peritoneal cavity of a non-menstruating species, or changing genetic features, will not reflect all pathophysiological aspects of human endometriosis but might still provide invaluable information.
For more information on the “best-fit” rodent models see https://www.frontiersin.org/articles/10.3389/fphys.2021.806574/full
Non-human primate models
Some non-human primates have cyclic menstrual periods and can spontaneously develop endometriosis, although it occurs with a significantly lower frequency than in humans. Taking into account their similarity to humans regarding phylogenetics, reproductive physiology and anatomy, and the presence of spontaneous endometriosis histologically and macroscopically identical to its human counterpart, non-human primate may represent the best animal models in endometriosis research.
Considering the limitations of the rodent models in representing all aspects of the disease, researchers have turned to nonhuman primates in the past and present. However, ethical considerations and laborious requirements for the maintenance of these primate animal models have limited their use in research and inhibited the progression of potential drug developments.
The first non-human primate model was established by Te Linde and Scott in 1950 using an autologous transplantation in rhesus monkeys. After that, several other non-human primate models were developed to study endometriosis using the Japanese macaque, the pigtailed macaque, the baboon, and the cynomolgus monkey.
Non-human primate models that spontaneously develop endometriosis or that have been intraperitoneally transplanted with endometrium have contributed to investigating the pathogenesis and treatment of endometriosis and are still occasionally used for the development and optimization of drug candidates.
Although spontaneous endometriosis could be considered the best possible model, moderate-severe disease is not commonly found in nonhuman primates. Diagnosis of spontaneous endometriosis in nonhuman primate models is difficult, since (as in humans) there is a lack of reliable non-invasive methods for the early detection of the condition, and a large number of animals is required.
For more information on primate models of endometriosis : https://www.jstage.jst.go.jp/article/tjem/226/2/226_2_95/_pdf/-char/en
Other animal models
There are other animals that are also used to model endometriosis such as chickens, rabbits, sheep and cows. The studies using these models frequently focus on a particular physiopathological aspect.
Chickens have been used for disease mechanism studies notably and rabbits and cows have been used to model key points of endometriosis-associated infertility.
The chicken chorioallantoic membrane (CAM) model can be considered as an animal model in the broader sense. Fragments of human endometrial tissue are cultured on the basal layer of the CAM of fertilized chicken eggs after prior incubation for 7–10 days. Endometrial fragments invade across the epithelium into the mesenchymal layer and develop endometriosis-like lesions in this layer of the CAM within 3 days.
The CAM assay is suited for the investigation of mechanisms involved in invasion and angiogenesis during the first steps in the establishment of endometriotic lesions. The ectopic lesions are easily accessible and can be simply monitored. However, this model cannot help investigate the immunological or inflammatory responses or analyse long-term drug effects.
Non-animal models
Cell cultures are an indispensable tool to help uncover fundamental biophysical and biomolecular mechanisms by which cells assemble into tissues and organs, how these tissues function, and how that function becomes disrupted in disease. Findings that have emerged from in vitro observations have led to a whole new group of drugs for endometriosis treatment.
In endometriosis research, cells collected from biopsies and culture on two-dimensional surfaces, have generated hundreds of publications. Co-culture models, in particular, in which two of more different cell types are culture together, often separated by a fluid-permeable membrane, have been used to assess the effect of one cell type on another cell and how they interact.
However, these 2D models lack important features for cellular function, including their original microenvironment, their tissue-specific architecture, and blood flow perfusion. In the quest to create better models to observe cell to cell interaction, researchers have developed 3D cell models of endometriosis, with more realistic biochemical and biomechanical microenvironments. These organoids aim to provide better phenotype and gene expression, further opening the possibility to test interactions among different cells found in endometriotic lesions.
Improvement in genetic tools
Endometriosis is thought to be partly caused by genetic factors. Genomic wide association studies that emerged more than a decade ago are an important tool to study complex genetic diseases such as endometriosis.
GWAS can help infer causality and uncover pathogenic mechanisms for endometriosis. To date, GWAS revealed more than 10 genomic regions associated with endometriosis. This information has allowed new insights into the biological pathways that could be associated with the pathology of endometriosis.
Besides helping identify new potential therapeutic targets, these types of studies may be helpful in dissecting the different phenotypes and genotypes of the disease which may lead to novel transgenic animal models that may provide new insights into the disease.
Last edited: 13 June 2023 15:42