Hypertension: elevated blood pressure
Hypertension, also known as elevated blood pressure, is the most common chronic disease in the world. Although they may not realise it, as many as 1 in 3 adults in the UK have high blood pressure.
Symptoms are most often unnoticeable, but if left untreated, they increase risk of a number of serious and potentially life-threatening health conditions such as heart failure, aneurysms and even kidney disease. High blood pressure is responsible for more than half of all strokes and heart attacks. High blood pressure puts extra strain on the blood vessels, heart and other organs, such as the brain, kidneys and eyes which can lead to substantial morbidity and mortality. In the UK, high blood pressure is the third biggest risk factor for all disease after smoking and poor diet. The only way to find out if ones blood pressure is high is to have ones blood pressure checked.
The precise cause of elevated blood pressure often cannot be determined. Risk factors for primary (formerly called essential) hypertension include advancing age, obesity, high dietary salt consumption, and low dietary potassium intake, although these appear to contribute to, but not cause, hypertension. Secondary causes may involve the renal vasculature, endocrine organs, and kidney and these may be involved in up to 20% of cases of resistant hypertension. An increasing number of drugs used to treat cancer and other conditions are now recognised as causing hypertension, which is often severe.
A number of hypertensive subtypes also exist, and although they may make up only a small fraction of the cases of hypertension, they can nonetheless be relatively common, given the broad prevalence of hypertension itself. Over the past 20 years, some the most important scientific breakthroughs have emanated from the discovery of the basis of rare subtypes of human hypertension, including the genetic background of some forms of the disease.
The diversity of animal models of hypertension
Human essential hypertension is a complex multifactorial disease which is influenced by genetic and environmental factors. As a multifactorial and systemic disease that involves multiple organs and systems, hypertension remains a challenging disease to study.
Development of experimental models of hypertension allowed dissection and isolation of various factors associated with regulation of blood pressure, inheritance of hypertensive traits, and cellular responses to injury. In doing so, these models are beneficial in the pharmacological screening of potential antihypertensive drugs. They have help researchers elucidate multi-faceted factors that regulate blood pressure including those involving environmental, genetic/epigenetic, physiological and microbial factors. To obtain these results, researchers have used different animal models where blood pressure is controlled by a diverse range of mechanisms.
Although excellent animal models with good human fidelity have been developed for many of the rare causes of hypertension, models of primary hypertension have been more difficult to develop, largely because the causes of the human disorder are unclear.
In fact, one of the main challenges that researchers have had to face is the lack of a unique model that appropriately emulates all of the different components that make up the complex phenotype of essential hypertension. The traditional way to tackle this conundrum has been to break down the multifactorial aspect into individual and isolated components, understand how each component functions and make inferences on how such functions affect the complete system. The overall aim of this approach is to place all the pieces of the puzzle back together to construct a network that can then be targeted therapeutically.
Over the years, researchers developed many different ways to cause hypertension in animals in order to identify the different molecular and cellular pathways at play. However, none of the animal models developed were capable of reflecting the entire situation that occurs in humans. In other words, several animal models are required to examine particular cardiovascular changes in an effective study. Each of the studied models adds to the complexity of the picture of hypertension.
Several criteria need to be considered in order to develop an ideal animal model for hypertension. These factors include the feasibility and size of the animals, the reproducibility of the model, the ability to predict the potential antihypertensive properties of a drug, the similarity to human disease (mode of the disease: slow on-set versus acute), and economical, technical, and animal welfare considerations.
The different animal models of hypertension
Over the years, various ways of causing hypertension have been used to develop animal models of the disease that mimic hypertensive responses observed in humans. Many of these use the same etiological factors that have been hypothesized to contribute the disease in humans such as excessive salt intake, hyperactivity of renin-angiotensin-aldosterone system (RAAS), and genetic predisposition.
Different pharmacological, environmental and genetic models using different animal species have provided useful and valuable information on the aetiology, pathophysiology and complications of human cardiovascular and metabolic disorders, and a platform to examine the efficacy of pharmacotherapy. Despite the common mechanism of RAAS activation in hypertension across different species, there are some species differences that need to be considered. A major limitation of these experimental models remains notably the anatomical differences between these animal species and humans. Species differences must be carefully taken into consideration to ensure the safety of newly developed pharmacological agents.
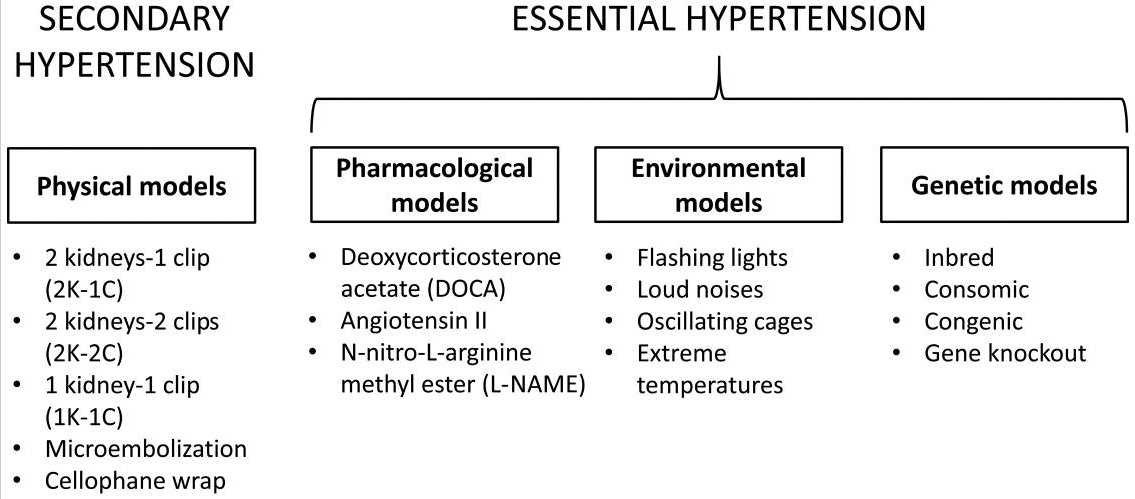
- induced models of hypertension : Physical models
Some of the earliest animal models of hypertension involved the moderate constriction of renal arteries (Goldblatt kidney) with a clip, allowing for an elevated systolic blood pressure without any effect on renal function, but also the ablation of the parenchyma (Page kidney). Both used larger animals such as dogs and non-human primates in the 1930s.
Subsequent models using rats, rabbits, sheep and cats were developed. Currently, the preferred animal model for this system is the rat. Along with rats, occasionally mice, monkeys, and pigs are also used as a model for experimental hypertension. However, these species have not been studied extensively for both practical and financial reasons.
The pathophysiology of the kidney-clip models mimicking renal arterial stenosis closely mimicked their human analogues, however, renovascular hypertension and Page kidney represent only a small fraction of human hypertension.
- induced models of hypertension : Pharmacological
It is also possible to induce hypertension in animals using drugs. Mineralocorticoids or their synthetic derivatives, including deoxycorticosterone acetate (DOCA), are used with sodium chloride in unilateral nephrectomised rats to induce hypertension. These molecules cause the suppression of renin and fluid reabsorption is increased, thereby producing a low renin-volume overload model of hypertension. Rats are the animal of choice for this procedure but a DOCA-hypertensive Yucatan miniature swine model was also made, which doesn’t require excess dietary salt for sustaining hypertension.
Using this pharmacological model, key sodium-independent mechanisms for mediating hypertension, including upregulation of Ang-II receptors in the central nervous system, elevated vasopressin, increased oxidative stress and endothelin, have been identified. Aside from elucidating the molecular mechanisms underlying renal hypertension, it provides a useful platform for investigating the natural history of disease, including any complications.
Glucocorticoids can also be used to induce hypertension but this approach tends to be less effective than the DOCA-salt method. An alternative is chronic infusion of RAAS components, including Ang-II or using nitric oxide (NO), a potent vasodilator derived from the intact endothelium, production by NOS.
- induced models of hypertension : Environmental/diet
In humans, hypertension can be in part caused by environmental factors such as diet. The same can be said for animal models. Environmental stress, including separate or simultaneous introduction of flashing lights, loud noises and oscillating cages (55–57), or long-term exposure to high salt, fat or sugar in the diet, can be used to induce hypertension in animal models. In particular, oxidized frying oil is considered a risk factor for hypertension, causing hypertension in rats after chronic consumption of heated oil diets. Extremes of temperature, particularly coldness, can also induce a hypertensive phenotype, as observed in rats exposed to 5°C for 3 weeks.
In these models, it is likely that the environmental factors increase activity of the sympathetic nervous systemand the RAAS activation, which is responsible for triggering hypertension .
- Genetic models of hypertension
Genetic factors are estimated to influence up to 50% of blood pressure variability in essential hypertension. In 2000, the millennium genome project for hypertension was initiated to identify genetic variants that predispose individuals to hypertension. The project partly fed the development of numerous genetic models using different species, which provided insights into the physiological mechanisms of hypertension.
- Inbreeding
Genetic models of hypertension were first produced through inbreeding, sibling mating of hypertensive rats over several generations to produce strains with genetic homogeneity when compared with the reference control group.
In 1963, Okamoto and Aoki introduced through breeding of Wistar rats with their sisters, a spontaneous experimental hypertension model without the involvement of physiological, pharmacological, or surgical intervention. This model is known as the spontaneously hypertensive rat (SHR), which is the genetic strain of hypertensive rat. In SHRs, increases in systolic blood pressures to 180–200 mmHg after 4 weeks of growth were observed, compared with the Wistar-Kyoto rats (WKY) that remain normotensive. SHR has notably become the model of choice to determine the genes responsible for hypertension, to evaluate complications of target organs and for the screening of antihypertensive agents. It has been a cornerstone of medical research in experimental hypertension.
In the 1970s, Lewis Dahl discovered that some rats became hypertensive when given a normal salt diet. He selectively bred them to produce the Dahl salt-sensitive rat strain and its control strain the Dahl salt-resistant. Dahl salt-sensitive strain rats fed with a high-salt diet (8% NaCl) develop particularly severe hypertension. The Sabra strain also demonstrates salt-sensitive hypertension.
At around the same time, Schlager and colleagues developed blood pressure high (BPH), blood pressure low (BPL) and normotensive blood pressure normal (BPN) strains of mice by crossing eight normotensive strains of mice. These strains of mice have firmly established themselves as the leading genetic strain of hypertension in the mouse and parallels the hypertension in SHR with both models developing elevated blood pressure at a very early age (Jackson et al., 2019).
- Transgenic strains
Transgenic technology can be used to investigate the specific role of different genes in the regulation of blood pressure. Broadly, within genetic models, there are monogenic models such as congenic (i.e., with mutations in a specific genetic back-ground or inbred strain), consomic (i.e., with a single entire chromosome from another strain) and transgenic models (i.e., genetically modified such as knockout strains).
For example, the mutant renin gene from mouse was transferred to rats, producing elevated Ang-II levels and hypertension, similarly, insertion of the human renin gene into mice also consistently demonstrated activation of genes involved in the RAAS .
More targeted gene knockout approaches have permitted selected and controlled disruption, including deletion, overexpression or subtle mutations, of a gene product. Conditional knockout with tissue- and time-dependent specificity has brought on information about the loss of a particular gene at specific time points or in particular organs. Global or cell-specific knockout models of single or multiple genes targets can be generated, and this has helped decipher the roles of genes and molecular pathways in complex polygenic-multifactorial diseases such as hypertension.
With the advent of advanced gene-editing techniques, such as CRISPR/Cas9 technology, knockout rodents have become a powerful tool to delineate gene functions. Gene knockout is often performed in mice because of the relative ease in introducing genetic mutations, and this has led to an increased understanding of different cardiovascular disorders with potential for translational application. This is still limited in larger animals, making this the single most important advantage of using rodent models of disease. These genetically modified rodents are an important tool for us to further understand the many different causes of essential and secondary hyper-tension.
Last edited: 15 May 2023 10:23
