Paul Ehrlich: a hundred years of chemotherapy - 1891-1991
Anthony S Travis, Sidney M Edelstein Center for history and Philosophy of science, technology and medicine. The Hebrew University of Jerusalem
Originally printed in The Biochemist 13(5). Reproduced with kind permission from the Biochemical Society, London.
 'NOW, ladies and gentlemen, I may perhaps take the liberty of inviting you to look into the workshop of the chemo-therapist' With these words, Paul Ehrlich (1854-1915) conveyed his audience at the 1913 Seventeenth International Congress of Medicine into the realms of a new subdiscipline.ANCHOR Although Ehrlich had already achieved fame, and the Nobel Prize in 1908, for his immunity studies, it was the chemotherapeutic behaviour of the anti-syphilis drug Salvarsan that elevated him to the role of scientific superstar after 1910.
'NOW, ladies and gentlemen, I may perhaps take the liberty of inviting you to look into the workshop of the chemo-therapist' With these words, Paul Ehrlich (1854-1915) conveyed his audience at the 1913 Seventeenth International Congress of Medicine into the realms of a new subdiscipline.ANCHOR Although Ehrlich had already achieved fame, and the Nobel Prize in 1908, for his immunity studies, it was the chemotherapeutic behaviour of the anti-syphilis drug Salvarsan that elevated him to the role of scientific superstar after 1910.
Collaboration between Science and Industry
Ehrlich's reputation derived from a novel approach to biological problem solving that used the industrial products and theories of organic chemistry. He had been successful in the classification of synthetic dyestuffs as stains, in their use for investigating cellular combustion, in adapting theories of dyeing to develop models for both combustion and immunity, and in exploiting the properties of dyes and their close analogues in the development of chemo-therapy. He moved from one area of investigation to the next as soon as he had gone as far as he could go, given the materials, theories, and investigative instruments and methods of the day. At each move he was better armed with experience to tackle new problems. This endowed him with the powers of persuasion that did much to bring about acceptance of often complex ideas.
Ehrlich also became a master at persuading other audiences, especially those who would provide financial backing: the dyestuffs manufacturers who were eager to diversify; the Prussian ministers of health, interested in a healthier working population and greater social stability; and the Colonial Office, whose aspirations were con-strained by unconquerable tropical diseases.ANCHOR,ANCHOR
Ehrlich's 1913 address was a retrospective appraisal of the final stage of his career, and a testimony to his remark-able successes in tackling practical puzzles, most especially the adaptation of ideas from the well established discipline of chemistry to give legitimacy to the theoretical basis of immunity and chemotherapy. This article deals with Ehrlich's involvement with chemo-therapy, especially during the period 1900 to 1910, when he used the chemical products provided by two key manu-facturers: Meister, Lucius & Briining (or the Hoechst dyeworks) and Cassella & Co, of Mainkur, both situated near Frankfurt.
The Role of Dyestuffs
From 1880 there was intense competition in the synthetic dyestuffs industry, especially in Germany, the world leader.ANCHOR Some intermediates for dyes were converted into compounds that acted as pain killers and anti-depressants, even at this early stage of drug development. In 1883, the board of the Hoechst dyeworks authorized the construction of a laboratory for drug research, managed by an organic chemist, and modelled on a dyestuffs research laboratory. The Bayer company of Elberfeld was also interested in new medicinal products. Research and development, as at Hoechst, was organ-ized on the lines newly introduced into the dye industry.
In the early 1890's, the Hoechst company took up the production of drugs for serum therapy.ANCHOR Serums were produced, based on Robert Koch's work with tuberculin (1892), and Ehrlich's studies on diphtheria (1894). Then, in 1901, Ehrlich began to concentrate on what in 1904 he called 'chemotherapy' using dyes of the benzopurpurine, acridine and triphenyl-methane (magenta or fuchsine) classes of compound which were placed at his disposal by the Cassella and Hoechst companies. Dyestuff compounds were no strangers to Ehrlich; they had been familiar to him almost from the moment that he had embarked on his medical studies.
Ehrlich used synthetic dyestuffs as staining agents during studies for his doctoral thesis in medicine at Strasbourg during 1873-74. He drew parallels between the nature of dye attachment to fibre and to living tissue, borrowing the latest theories of textile dyeing.ANCHOR
The studies were extended when Ehrlich turned to the poorly understood physiological processes which were seen to arise when dyestuffs were injected into animals. The dyestuffs underwent loss of colour during reduction, affording their leuco forms, and regained colour during oxidation. The results were explained through a striking analogy drawn with the theory of Otto N. Witt that assigned the quite separate functions of colour rendering and colour fixing to specific functional groups in dyestuffs.ANCHOR
Improved staining techniques, and novel dyes, led to other advances. The dyestuff methylene blue was particu-larly important to Ehrlich, since it was shown to be a good stain for bacteria (1881), and to have specific affinity for nerve cells (1886). Ehrlich suggested to chemists, notably Heinrich Caro at the BASF (Badische Anilin und Soda Fabrik) company, that a chemically modified form of methylene blue might provide the clues to its selective action. The result was rhodamine, which demonstrated that selective staining was a function of the sulphur atom in methylene blue, and which rewarded the dyestuffs industry with the profitable indamine series of compounds.ANCHOR In this way a mutually supportive relation-ship grew up between the developing theoretical biology of the time, and the increasingly powerful dyestuffs industry. Methylene blue played an equally important role in the first ideas about the applications and mechanisms of chemo-therapy.
Ehrlich's first steps towards chemo-therapy began exactly one hundred years ago, in 1891, when he noted a report that methylene blue was an effective stain for the parasite of malaria. This caused him to test its action as a treatment to eliminate the infection.
Although the results showed promise, a major drawback was the absence of an effective means for transmitting and maintaining parasitic organisms in suitable animals. This work soon gave way to experimentation with the new anti-toxins of von Behring—and the study of specific immunity against diphtheria and tetanus.
In 1896, The State Institute for Serum Research and Serum Control was opened at Steiglitz, near Berlin, with Ehrlich as director (figure 1). There he established methods for measuring anti-toxic activity of serum, with the outcome that optimal doses could be prescribed. The results were placed on a quantitative footing by defining an arbitrary unit of toxin, and from this the strength of the remedial dosage could be calculated. Moreover, the antitoxin behaviour was explained through a convincing pictorial representation of the combining and toxic components of antigenic toxin. These were called the haptophore and toxophore groupings respectively, and, again, were based on Witt's chemical ideas. This became Ehrlich's side chain theory.ANCHOR (See figure 2)
Serum therapy failed to control a number of infectious diseases, and this led to renewed interest in the use of synthetic chemicals for chemotherapy. The endeavour was assisted by a pre-meditated change of locale. In 1898, Ehrlich's institute was transferred to the newly opened Institute for Experimental Therapy in Frankfurt, close to the works of the Hoechst and Cassella companies. This was five years before the extensive immunity studies, that had consumed Ehrlich's efforts since 1891, came to a close. However, he was already considering the possibility of new types of biomedical products.ANCHOR From around 1903, therapy with synthetic dyestuffs and other chemical products from the local dye companies became the most promising area for laboratory investigation. This activity was expanded when Ehrlich's institute was joined by a new research centre, the Georg Speyer Haus, opened in 1906. The enterprise lasted until 1913, and was made possible because a method had become available for transmitting pathogenic trypanosomes to experimental animals and of maintaining their levels in the blood.
The Japanese Connection
 Biomedical research was assisted by many factors and personalities, not all of them of European origin. Japanese specialists made particularly important contributions. Their interest in western science and technology grew during the Meiji era (1869-1912), and several medical institutes, modelled on German lines, were opened in Japan. Another important side to the Japanese interest in biological and chemical studies was improvement in agricultural science. By the end of the nineteenth century, Japanese students could be found in Paris, Manchester, Berlin, Frankfurt and the United States.
Biomedical research was assisted by many factors and personalities, not all of them of European origin. Japanese specialists made particularly important contributions. Their interest in western science and technology grew during the Meiji era (1869-1912), and several medical institutes, modelled on German lines, were opened in Japan. Another important side to the Japanese interest in biological and chemical studies was improvement in agricultural science. By the end of the nineteenth century, Japanese students could be found in Paris, Manchester, Berlin, Frankfurt and the United States.
Kitasato was one of the most prominent Japanese to venture to Europe. He worked at the Pasteur Institute in Paris, with Ehrlich, and with von Behring. On his return to Japan, Kitasato set up the Institute for Infectious Diseases in Tokyo, and dispatched promising scholars to the laboratories of Pasteur, Koch, and Ehrlich. Kyoshi Shiga was Ehrlich's first Japanese assistant, arriving for a three year stint in 1902.
Trypan Dyes
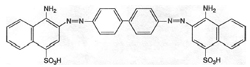 Shiga began with experiments using azo dyes of the benzopurpurine type, notable because they coloured cotton without the aid of mordants (Figure 3). In 1903, Ludwig Benda of Cassella & Co. increased the solubility of one of the benzopurpurine dyes by sulphonation, an established method of improving the applicability of dyestuffs. The soluble product, also more useful to the studies of an effective therapy, was later named trypan red. The first studies, in which the suppression of sleeping sickness was reported, were published jointly with Ehrlich in 1904. In the same year Cassella & Co. joined forces with Hoechst as part of a major trading partnership. This enhanced their roles in advancing systemic therapy with chemicals. Benda prepared the yellow acridine dye trypa-flavin for Ehrlich, which gave good results on trypanosome infections. However, it lacked the strength of trypan red.
Shiga began with experiments using azo dyes of the benzopurpurine type, notable because they coloured cotton without the aid of mordants (Figure 3). In 1903, Ludwig Benda of Cassella & Co. increased the solubility of one of the benzopurpurine dyes by sulphonation, an established method of improving the applicability of dyestuffs. The soluble product, also more useful to the studies of an effective therapy, was later named trypan red. The first studies, in which the suppression of sleeping sickness was reported, were published jointly with Ehrlich in 1904. In the same year Cassella & Co. joined forces with Hoechst as part of a major trading partnership. This enhanced their roles in advancing systemic therapy with chemicals. Benda prepared the yellow acridine dye trypa-flavin for Ehrlich, which gave good results on trypanosome infections. However, it lacked the strength of trypan red.
The Bayer Company also actively promoted therapeutic researches, sponsoring Felix Mesnil and Maurice Nicolle at the Pasteur Institute from 1906. They applied blue and violet benzopurpurine dyes with considerable effect. However, although Afridol blue (trypan blue) attacked trypanosomes in cattle, the animals also turned blue, which did little to promote the sale in butcher's shops. Afridol violet was a similar dye tested at the Pasteur Institute, but the results were no more promising.
From 1904 there was a second main thrust in Ehrlich's research, when arsenic containing compounds were introduced. The first arsenic product investigated was atoxyl.
Atoxyl was marketed from 1902 and was tried by Koch against African sleeping sickness, and, in 1905, by Paul Uhlenhuth against fowl spirilloses. Uhlenhuth established the therapeutic action of arsenicals against diseases caused by protozoa, and observed some action against trypanosome infections. However the results were not good, especially as the patient's vision was affected. The first successful use of atoxyl against a trypanosome was reported from the Liverpool Institute for Tropical Diseases in 1905 by Thomas and Breinl. Ehrlich expressed a great interest in their results and began to employ atoxyl. However, in vitro and in solution, there was no effect on parasites. Perhaps remembering his studies on combustion, and the ability of dyes to undergo reduction, Ehrlich resorted to reduction of atoxyl, in 'which arsenic is pentavalent, so that it became trivalent'. Tried in vitro, the reduced product was effective against trypanosomiases.ANCHOR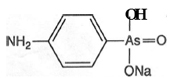 The chemical constitution of atoxyl was uncertain, and this problem was taken up by Alfred Bertheim in the spring of 1906, shortly after he arrived at the Georg Speyer Haus. His results were published with Ehrlich during the following year and showed that atoxyl was the sodium salt of p-amino-phenylarsonic acid (see figure 4). Because this work established the presence of a free amino group, atoxyl could be modified, and large scale laboratory synthesis of derivatives was undertaken by Benda. Some gave promising results when tried in the tropics.
The chemical constitution of atoxyl was uncertain, and this problem was taken up by Alfred Bertheim in the spring of 1906, shortly after he arrived at the Georg Speyer Haus. His results were published with Ehrlich during the following year and showed that atoxyl was the sodium salt of p-amino-phenylarsonic acid (see figure 4). Because this work established the presence of a free amino group, atoxyl could be modified, and large scale laboratory synthesis of derivatives was undertaken by Benda. Some gave promising results when tried in the tropics.
Meantime, in Berlin during 1905, Fritz Schaudinn and Erich Hofmann discovered the microbe that caused syphilis. The Spirochaeta pallida of syphilis were observed to be similar in appearance to the much studied trypanosomes. Hofmann asked Ehrlich for potential experimental drugs, and the latter drew attention to the earlier experiments of Browning and Shiga with trypan red at the Georg Speyer Haus. Benda prepared a new hydroxy-nitrophenylarsonic acid compound and sent it to the Georg Speyer Haus, but, inadvertently, it was not used. Knowing nothing of this, Ehrlich and Bertheim prepared the same compound and reduced it. 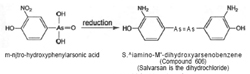 The outcome was the sixth compound in the series commencing 600. It was a condensation product, 3,3'-diamo-4,4'-dihydroxyarseno-benzene, containing trivalent arsenic, and structurally analogous to an azo compound (see figure 5). The preparation of no. 606 was made up in May 1909, around the time that Sahaschiro Hata joined Ehrlich (figure 6).
The outcome was the sixth compound in the series commencing 600. It was a condensation product, 3,3'-diamo-4,4'-dihydroxyarseno-benzene, containing trivalent arsenic, and structurally analogous to an azo compound (see figure 5). The preparation of no. 606 was made up in May 1909, around the time that Sahaschiro Hata joined Ehrlich (figure 6).
Hata finds the Magic Bullet
Sahaschiro Hata found that a single injection of compound 606 was sufficient to overcome chicken spirillosis. Relap-sing fever, which had resisted the arsenicals, was also cured. Hata had con-siderable experience of transmitting syphilis to animals by inoculation and tried numerous arsenic compounds on infected rabbits. In June, treatment of syphilis was successful. Tests on humans were conducted. The new substance showed great promise, though there were unpleasant side-effects and fatalities, and the cure was not brought about by a single dose. The remarkable drug was patented in Germany during June 1909, and in Britain almost exactly one year later. The breakthrough was announced at the Congress for Internal Medicine, Wiesbaden, in April 1910. The first treatment of patients, by J. Iversen of St. Petersburg who cured relapsing fever with 606, were described, along with many other successful results.ANCHOR The Georg Speyer Haus went on to distribute 65,000 doses of compound 606 to 500 physicians for clinical trials by the end of 1910.
The breakthrough was announced at the Congress for Internal Medicine, Wiesbaden, in April 1910. The first treatment of patients, by J. Iversen of St. Petersburg who cured relapsing fever with 606, were described, along with many other successful results.ANCHOR The Georg Speyer Haus went on to distribute 65,000 doses of compound 606 to 500 physicians for clinical trials by the end of 1910.
The Hoechst factory sent two chemists to the Georg Speyer Haus to assist with the scaling up. In December 1910, Hoechst completed installations for manufacture of 606, undertook trials, and began to market the product. It was soon generally known as Salvarsan (the dichloride salt) or Arsphenamine. Synthesis at the Georg Speyer Haus ceased, but Ehrlich's institute received a share of the profits, in accordance with earlier contractual arrangements made with Hoechst and Cassella.
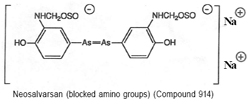
The major problem with Salvarsan was its poor solubility. In 1912, this was overcome with the preparation of Neo-salvarsan (no. 914) from the reaction of Salvarsan with sodium formaldehyde sulphoxylate (see figure 7). Neo-salvarsan was easier to manufacture, requiring fewer precautions. It became the most widely used anti-syphilitic agent and remained in use until the 1940s. For the Hoechst dye works, Salvarsan represented one eighth of turnover, amounting to some £50,000 of sales in 1911, a figure that was soon trebled after Neosalvarsan had entered the market place.
The Explanation of Drug Action
 Ehrlich was very concerned with a theory of drug action, but difficulties in arriving at a mechanism came from a conflict between physical (physico-chemical) and chemical viewpoints.ANCHOR Ehrlich supported the latter, and suggested that both drugs and poisons form chemical bonds with the cell. The drug, according to this picture, 'fits its biological receptor'.
Ehrlich was very concerned with a theory of drug action, but difficulties in arriving at a mechanism came from a conflict between physical (physico-chemical) and chemical viewpoints.ANCHOR Ehrlich supported the latter, and suggested that both drugs and poisons form chemical bonds with the cell. The drug, according to this picture, 'fits its biological receptor'.
Ehrlich again drew analogies with dyestuffs. He postulated that drug action was similar to that of antitoxins. Therapy was possible 'only when the arsenic group is in the unsaturated form, corresponding to the trivalent radical.' There was a strong suggestion of bonding:'these unsaturated compounds contain unsatisfied avidities (valencies) which render them capable of additive reaction with other compounds'.ANCHOR This was the basis of Ehrlich's chemoreceptor theory, developed after observations on strains that showed an acquired resistance to dyestuffs.
Steric factors were also invoked to explain the action of the drug, or arseno-receptor, by analogy with ortho condensation reactions in chemistry. The arseno-receptor was seen to act as a clamp for o-quinones, and had such a range of chemical affinities that it could seize and hold 'a yet unknown number of purely organic dyestuffs.'
Ehrlich defined an ideal medicament as one that would contain a haptophore group to enable it to attach specifically to the receptor on the parasite, and that would be harmless to the host 'because it was not anchored by the organs. It would however strike the parasites with full force,' and thereby correspond to the immune substances. Just as antibodies 'in the manner of magic bullets, seek out the enemy,' it was hoped that drugs might be equally accurately targeted and score a 'bullseye' in this way.
Ehrlich's much cherished therapia sterilans magna, the cure of the body by means of a single injection, was not realized. Nevertheless, the 'siege of a bacterial fortress' was shown to be possible with chemical products based upon dyestuffs as models. The hope of more widespread applicability was chemistry-inspired theories based on the chemical nature and reactivity of dye-stuffs.
Références
- Ehrlich, P. (1959-60). Papers reprinted in The Collected Papers of Paul Ehrlich (Editors F. Himmelweit, M. Marquardt and H. H. Dale), vol III. London: Pergamon. 1913. 'Chemo¬therapy'; 3, 505-518.
- Lenoir, T. (1988). 'A Magic Bullet: Research for Profit and the Growth of Knowledge in Germany around 1900,' Minerva 26, 66-88.
- Liebenau, J. (1990). 'Paul Ehrlich as a Com¬mercial Scientist and Research Adminis¬trator,' Medical History 34, 65-78.
- Travis, A. S. (1992). The Rainbow Makers: The Origins of the Synthetic Dyestuffs Industry in Western Europe. Lehigh University Press, Bethlehem, Pa.
- Hoechst (1913). Farbwerke vorm. Meister, Lucius und Bruning, 1863-1913. Hoechst: Fabwerke Hoechst.
- Travis, A. S. (1989). 'Science as Receptor of Technology Paul Ehrlich and the dyestuffs industry'. Science in Context, 383-408.
- Witt, O. N. (1876). 'Zur Kentiss des Baues und der Bildung farbender Kohlenstoff-verbindungen,' Berichte der Deutschen Chemischen Gesellschaft 9, 522-527.
- Ehrlich, P. (1959-60). Papers reprinted in The Collected Papers of Paul Ehrlich (Editors F. Himmelweit, M. Marquardt and H. H. Dale), vol II. London: Pergamon.1906. "Address Delivered at the Dedication of the Georg Speyer Haus'; 3, 53-63.
- Witkop, B. (1982). 'Paul Ehrlich: His Ideas and His Legacy', in Science, Technology and Society in the Time of Alfred Nobel (Editors C. G. Bernhard, E. Crawford & P. Sorbom). Oxford: Pergamon.
- Ehrlich, P. (1959-60). Papers reprinted in The Collected Papers of Paul Ehrlich (Editors F. Himmelweit, M. Marquardt and H. H. Dale), vol I. London: Pergamon. 1898. 'The Relations Existing Between Chemical Constitution, Distribution and Pharmaco¬logical Action'; 1, 596-618.
- Ehrlich, P. (1959-60). Papers reprinted in The Collected Papers of Paul Ehrlich (Editors F. Himmelweit, M. Marquardt and H. H. Dale), vol III. London: Pergamon. 1913. 'Chemo¬therapy'; 3, 505-518.
- Ehrlich, P. & Hata, S. (1910). Die experimented Chemotherapie der Spirillosen. Berlin: Julius Springer.
- Parascandola, J. & Jasensky, R. (1974). 'Origins of the Receptor Theory of Drug Action,' Bulletin of the History of Medicine 48, 199-220.
- Ehrlich, P. (1959-60). Papers reprinted in The Collected Papers of Paul Ehrlich (Editors F. Himmelweit, M. Marquardt and H. H. Dale), vol III. London: Pergamon. 1913. 'Chemo¬therapy'; 3, 505-518.
Last edited: 27 August 2014 06:06
