Leukaemia
 Leukaemia (or leukemia) is a form of blood cancer caused by the abnormal growth of blood cells. It is commonly associated with children, as it is one of the few cancers that are regularly seen at this age, although leukaemia is far more common in the elderly. Approximately 350,000 people are diagnosed with leukaemia each year, with 250,000 deathsANCHOR.
Leukaemia (or leukemia) is a form of blood cancer caused by the abnormal growth of blood cells. It is commonly associated with children, as it is one of the few cancers that are regularly seen at this age, although leukaemia is far more common in the elderly. Approximately 350,000 people are diagnosed with leukaemia each year, with 250,000 deathsANCHOR.
Most leukaemias are either myeloid or lymphocytic/lymphoblastic, which respectively affect the myeloid cells, which produce red blood cells, and the lymphoid cells, which produce white blood cells. Both types can be either acute, where a rapid increase in abnormal, immature white blood cells crowd out normal blood cells in the bone marrow, or chronic, where the build-up is slower and consists of mostly mature blood cells. The most common forms are acute myeloid and chronic lymphocytic, each accounting for roughly 30% of cases. Approximately 17% of leukaemias do not fit into this categorisation.
Unlike most cancers, leukaemia often arises from well-understood, relatively simple genetic mutations. This has allowed researchers to develop treatments that have dramatically changed the fate of patients, although this varies significantly depending on the type of the disease.
Acute myeloid leukaemia
Chronic myeloid leukaemia
Acute lymphoblastic leukaemia
Chronic lymphocytic leukaemia
References
Acute myeloid leukaemia
The five-year survival rate for AML in people over 65, the most common demographic, is less than 10%. It is similar to solid cancers, such as breast or colon cancer, in that each person and even each cell hosts a different combination of dozens of mutations. It is this diversity that has made AML so difficult to treat relative to other leukaemias.
The standard treatment is chemotherapy, with blood stem cell transplant potentially available for healthier patients.
Now, researchers are working on a very different way of targeting the cancer. Since the 1960s scientists have noted that there are odd patterns of DNA methylation in leukaemia patients. Methylation is the process of adding a chemical tag, known as a methyl group, onto DNA. This can have a major effect and effectively controls whether a gene is switched on or off. This is a form of control known as epigenetics.
In 2004, the first drug to work epigenetically was approved to prevent leukaemia in patients with myelodysplastic syndrome, a non-aggressive cancer that progresses to AML in a third of casesANCHOR. 5-azacitidine (Vidaza) and decitabine (Dacogen) both interfere with DNA methylation.
In an attempt to find other targets for similar epigenetic drugs, researchers screened a library of RNA molecules designed to block the activity of epigenetic regulator proteins in a mouse model of AMLANCHOR. They discovered that one protein in particular, Brd4, was critical in maintaining the cancerous cells. A drug previously developed to block Brd4, JQ1, was used to show that it could be effective against a range of subtypes of AML. Brd4 works by ‘reading’ epigenetic marks known as acetyl groups and providing feedback to other proteins working to regulate DNA.
The genome of a patient with AML was sequenced for the first time in 2008, raising hopes for future targeted therapies. Since then, developments in sequencing technologies have greatly increased this potential. In 2013, researchers published results of sequencing DNA of 200 adults with AMLANCHOR. This revealed that more than three-quarters of them had a harmful mutation in an epigenetic enzyme. These enzymes are responsible for applying and removing chemical tags from DNA and DNA-associated proteins, through which they control expression and silencing of genes. Several of these enzymes, including DNMT3A, IDH1, IDH2, DOT1L and TET2, are now targets for drugs in development.
Chronic myeloid leukaemia
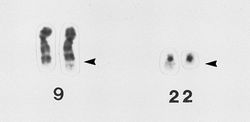 In 1960, researchers discovered that 95% of patients with chronic myeloid leukaemia (CML) have a chromosomal abnormality in the cancerous cells. This has since been known as the Philadelphia chromosome, named after the city in which it was discoveredANCHOR. It was later revealed that this was the result of parts of chromosomes 9 and 22 swapping places, which fuses two genes together known as BCR and ABLANCHOR. It was unclear whether this was caused by the cancer or a cause of the cancer until researchers inserted the BRC-ABL fusion gene into mice and saw that it led to a CML-like diseaseANCHOR ANCHOR ANCHOR ANCHOR. This led to work in trying to inhibit the activity of this gene.
In 1960, researchers discovered that 95% of patients with chronic myeloid leukaemia (CML) have a chromosomal abnormality in the cancerous cells. This has since been known as the Philadelphia chromosome, named after the city in which it was discoveredANCHOR. It was later revealed that this was the result of parts of chromosomes 9 and 22 swapping places, which fuses two genes together known as BCR and ABLANCHOR. It was unclear whether this was caused by the cancer or a cause of the cancer until researchers inserted the BRC-ABL fusion gene into mice and saw that it led to a CML-like diseaseANCHOR ANCHOR ANCHOR ANCHOR. This led to work in trying to inhibit the activity of this gene.
An inhibitor, later known as imatinib, for BCR-ABL was developed in 1996 following successful tests in cell cultures and miceANCHOR. Early clinical trials showed it to be a remarkable drug, causing complete remission in all patients. Imatinib was approved in 2001 and sold under the tradename Gleevec or Glivec. This was a turning point in CML treatment and the lifespan of patients has risen from 5-6 years to 10-20 years through imatinib and similar drugs.
Although imatinib is very effective, resistance is often a problem. Even at early stages in the disease, there can be a lot of genomic instability, which helps new mutations to form. Studies in mice suggest that the BCR-ABL protein itself promotes this instability ANCHOR. After eight years, about half of patients will have moved onto another drug after imatinib has become ineffectiveANCHOR. Fortunately there are several alternatives, including dasatinib, nilotinib, bosutinib and pontinib (approved in December 2012), which can get around several resistant mutations.
Acute lymphoblastic leukaemia
Acute lymphoblastic leukaemia (ALL) occurs mostly in children and accounts for 75% of childhood leukaemia cases. In the 1970s it killed around two-thirds of child patients ANCHOR, but as of 2005 ninety per cent of children under 15 are curedANCHOR.
Aminopterin was introduced in 1948 and became the first known compound to inhibit the growth of cancerANCHOR. It was able to cause temporary remissions in ALL in children. Aminopterin is an antifolate, which means that it blocks the metabolic pathways that use B9 folate vitamins. This particularly slows growth of bone marrow and in doing so the leukaemia goes into remission.
A year after aminopterin was demonstrated, a similar antifolate called methotrexate was shown to produce a drop in white blood cells in ratsANCHOR and prolong survival in mice with leukaemiaANCHOR. This later replaced aminopterin as the drug of choice, after tests in mice showing that it had fewer side effects than aminopterinANCHOR.
About 20% of ALL patients have the Philadelphia chromosome and so can also be treated with imatinib and similar drugs.
Patients are usually treated with chemotherapy to force the cancer into remission, which greatly reduces the number of cancer cells in the body. However, even a few cells remaining can allow the cancer to return. High doses of chemotherapy or total body irradiation will help to destroy nearly all of the cancer cells, but can also wipe out the haematopoietic stem cells in the bone marrow that produce blood cells. This has to be followed by a stem cell or bone marrow transplant from a donor in order to replace the lost cells.
A bone marrow transplant was used to cure leukaemia in 1985 for the first time. Since then, research has allowed bone marrow and stem cell transplants from donors who are not perfect matches to the recipient, which has greatly increased the opportunity for this therapy.
Development of hematopoietic stem cells is dependent on blood flow as shown after tests in zebrafish. Researchers tested over 2000 drugs on zebrafish to check their effects on stem cells development and found that those affecting blood flow increased productionANCHOR. Later research showed similar effects in mice and were able to test this by flowing liquids over stem cells in cell cultures. It is hoped that these drugs could be used to aid recovery in patients receiving stem cell transplants.
Chronic lymphocytic leukaemia
CLL can be treated with chemotherapy and monoclonal antibodies. It has a five-year survival rate of about 80%.
Some of those most exciting recent advances have been through reprogramming the patient’s T cells to attack and destroy the cancer. A sample of T cells can be genetically modified to produce a chimeric antigen receptor (CAR) that allows them to replicate rapidly in the body and target B cellsANCHOR ANCHOR. This receptor consists of an antibody, derived from a mouse monoclonal antibody, designed to recognise B cellsANCHOR. This destroys all of the patient’s B cells, both healthy and cancerous, which has serious side effects but also dramatic results in treating cancers that have resisted chemotherapy. Currently this is only available in small trials, and a key difficulty will be to scale it up to reach more patients.
This technology has developed over several decades from modifying mouse hybridoma cellsANCHOR ANCHOR to being able to modify the T cells taken from a mouse or a patientANCHOR ANCHOR. The first demonstration of human CAR T cells shrinking cancer was done in mice in 2002ANCHOR. It has since shown positive results in clinical trials for patients with CLLANCHOR, and has since also been used to treat relapsed ALL patientsANCHOR.
References
- Lozano R (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 Lancet 380(9859): 2095–128 doi:10.1016/S0140-6736(12)61728-0
- Kaminskas E, Farrell AT, Wang Y-C, Sridhara R, Pazdur R (2005) FDA Drug Approval Summary: Azacitidine (5-azacytidine, Vidaza) for Injectable Suspension The Oncologist 10(3):176–182 doi:10.1634/theoncologist.10-3-176
- Zuber J et al (2011) RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia Nature 478, 524–528 doi: 10.1038/nature10334
- The Cancer Genome Atlas Research Network (2013) New England Journal of Medicine 368, 2059-2074
- Nowell P, Hungerford D (1960) A minute chromosome in chronic granulocytic leukemia Science 132(3438):1497 doi: 10.1126/science.132.3438.1488
- Rowley JD (1973) A New Consistent Chromosomal Abnormality in Chronic Myelogenous Leukaemia identified by Quinacrine Fluorescence and Giemsa Staining Nature 243, 290-293 doi:10.1038/243290a0
- Daley GQ, Van Etten RA, Baltimore D (1990) Induction of chronic myelogenous leukemia in mice by the P210 BCR-ABL gene of the Philadelphia chromosome Science 247, 824–830
- Elefanty AG, Hariharan IK, Cory S (1990) BCR-ABL, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 9, 1069–1078
- Heisterkamp N et al. (1990) Acute leukaemia in bcr/abl transgenic mice Nature 344:251-253
- Kelliher MA et al (1990) Induction of a chronic myelogenous leukaemia-like syndrome in mice with v-abl and BCR-ABL Proc Natl Acad Sci USA 87, 6649-6653
- Druker BJ et al. (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells Nature Medicine 2, 561–566 doi:10.1038/nm0596-561
- Salloukh HF, Laneuville P (2000) Increase in mutant frequencies in mice expressing the BCR-ABL activated tyrosine kinase. Leukemia 14, 1401–1404
- Marin D et al (2012) Assessment of BCR-ABL1 Transcript Levels at 3 Months Is the Only Requirement for Predicting Outcome for Patients With Chronic Myeloid Leukemia Treated With Tyrosine Kinase Inhibitors J Clin Oncol 30, 232-238 DOI: 10.1200/JCO.2011.38.6565
- Steliarova-Foucher E, Stiller C, Kaatsch P et al (2004) Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study Lancet 364:2097–2105
- Hunger SP et al (2012) Improved Survival for Children and Adolescents With Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report From the Children's Oncology Group. Journal of Clinical Oncology
- Farber S, Diamond LK (1948) Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 238(23):787-93
- Franklin A, Belt M, Stoksstad E & Jukes T (1949) J Biol Chem 177, 621-629
- Burchenal J, Johnson S, Burchenal JR, Kushida M, Robinson E & Stock C (1949) Proc Soc Exper Biol Med 71, 381- 387
- Goldin A, Venditti JM, Humphreys SR, Dennis D, Mantel N, Greenhouse SW (1955) A quantitative comparison of the antileukemic effectiveness of two folic acid antagonists in mice J. Natl. Cancer Inst. 15 (6): 1657–64.
- North TE et al (2009) Hematopoietic Stem Cell Development Is Dependent on Blood Flow Cell 137(4):736–748 DOI: 10.1016/j.cell.2009.04.023
- Kalos, M et al (2011) T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia Sci Transl Med 3, 95ra73 DOI: 10.1126/scitranslmed.3002842
- Porter DL et al. (2011) Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia N. Engl. J. Med. 365:725-733 doi:10.1056/nejmoa1103849
- Sadelain M et al (2003) Targeting tumours with genetically enhanced T lymphocytes Nature Reviews Cancer 3, 35-45 doi:10.1038/nrc971
- Eshhar Z, Waks T, Gross G, Schindler DG (1993) Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724
- Stancovski I et al. (1993) Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J. Immunol. 151, 6577–6582
- Geiger TL, Jyothi MD (2001) Development and application of receptor-modified T lymphocytes for adoptive immunotherapy. Transfus. Med. Rev. 15, 21–34
- Ma Q, Gonzalo–Daganzo RM, Junghans R in Cancer Chemotherapy and Biological Response Modifiers, Annual 20 (eds Giaccone G, S. R. & Sondel P) 762 (Elsevier Science, New York, 2002)
- Brentjens R, Latouche JB, Riviere I, Sadelain M (2002) In vivo anti-tumor activity of genetically modified T cells is dependent on the method of ex vivo T cell expansion. Blood 100, 577a
- Porter DL et al (2011) Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia N Engl J Med 365:725-733 DOI:10.1056/NEJMoa1103849
- Brentjens R et al (2013) CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia Sci Transl Med 5, 177ra38 DOI: 10.1126/scitranslmed.3005930
Last edited: 5 November 2014 10:47
