Schistosomiasis
Schistosomiasis (also known as bilharzia) is caused by different species of the flatworm Schistosoma, each requiring a different freshwater snail intermediate host to complete its life cycle. S. mansoni and S. haematobium are predominantly human parasites whilst S. japonicum infects a wide range of domestic livestock as well as humans.
It is estimated that 780 million people are at risk of contracting the disease, with more than 200 million actual cases. Numbers continue to rise, despite the implementation of control programmes, due in part to the introduction of wide-scale irrigation schemes which help the snail host disperseANCHOR. Schistosomiasis is concentrated in sub-Saharan Africa with the highest prevalence and infection intensities usually found in school-age children and young adults; mortality in this region due to S. mansoni and S. haematobium is estimated at 280,000 deaths a yearANCHOR.
Disease control
Vaccine research
Immunopathology
Effects on concurrent infections
The future
Related links
Schistosomiasis is associated with poor socio-economic conditions and, most importantly, the lack of access to safe drinking water. People get infected when they enter water containing parasite larvae, released from the snail host, which penetrate their skin. The larvae must then migrate to the blood vessels surrounding the intestine (S. mansoni and S. japonicum) or bladder (S. haematobium), where they mature, mate and begin to lay eggs in the tissues.
Many eggs leave the body in faeces or urine (to continue the life cycle); those that lodge and accumulate in the tissues are the main cause of disease symptoms because they cause tissue scarring, which impairs organ function. Although schistosomiasis can be fatal, most infected individuals suffer from chronic debilitating symptoms such as anaemia, diarrhoea, fatigue, or impaired cognitive developmentANCHOR.
Disease control
Control strategies include access to adequate sanitation and clean drinking water; health education; snail control; and drug treatment of infected individuals or mass therapy. In areas of high disease prevalence, anti-schistosomal therapy offers the most cost-effective means for control but there are disadvantages. It can take several years for the symptoms of schistosomiasis to become apparent and the egg-induced tissue damage cannot be completely reversed simply by eradicating the worms. Moreover, drug treatment does not prevent rapid re-infection, and there is the ever present threat of the parasite developing resistance to the main agent in current use.
Vaccine research
A vaccine that provides protection against infection for an extended period is a powerful weapon for disease control (and ultimately eradication). As well as circumventing the risk of drug resistance, it would have the advantage of minimising a patient's chances of progressing to the advanced disease state. Most existing vaccines against infectious agents are based on the immune mechanisms that develop naturally after initial infection. Unfortunately, there appears to be little immunity to re-infection with schistosomes, especially in children – the group most at risk. As a consequence researchers have had to focus their efforts on immune responses in rodent and non-human primate models.
Vaccination with infective larvae of S. mansoni that have been weakened with ionising radiation induces a high degree of protection in both rodentsANCHOR and primatesANCHOR. Although this vaccine method raised hopes for the development of molecular vaccines, no single antigen has consistently induced equivalent protection, particularly when used in recombinant formANCHOR. The fact that schistosomes can live for many years in the hostile environment of the human bloodstream highlights their ability to evade the immune response.
To identify appropriate molecules that could be incorporated in a vaccine, researchers need to find the ones that represent the parasite’s 'Achilles Heel'; in other words, those that are vital for its normal biological functions or that it uses to protect itself.
Such molecules are likely to be released from the schistosome or located on its unique surface coat. There have been encouraging results on the latter; vaccination of mice with recombinant tetraspanin proteins that are present on the surface of both larval and adult stages resulted in an encouraging 60% reduction in 'worm burden' compared to controlsANCHOR.
Immunopathology
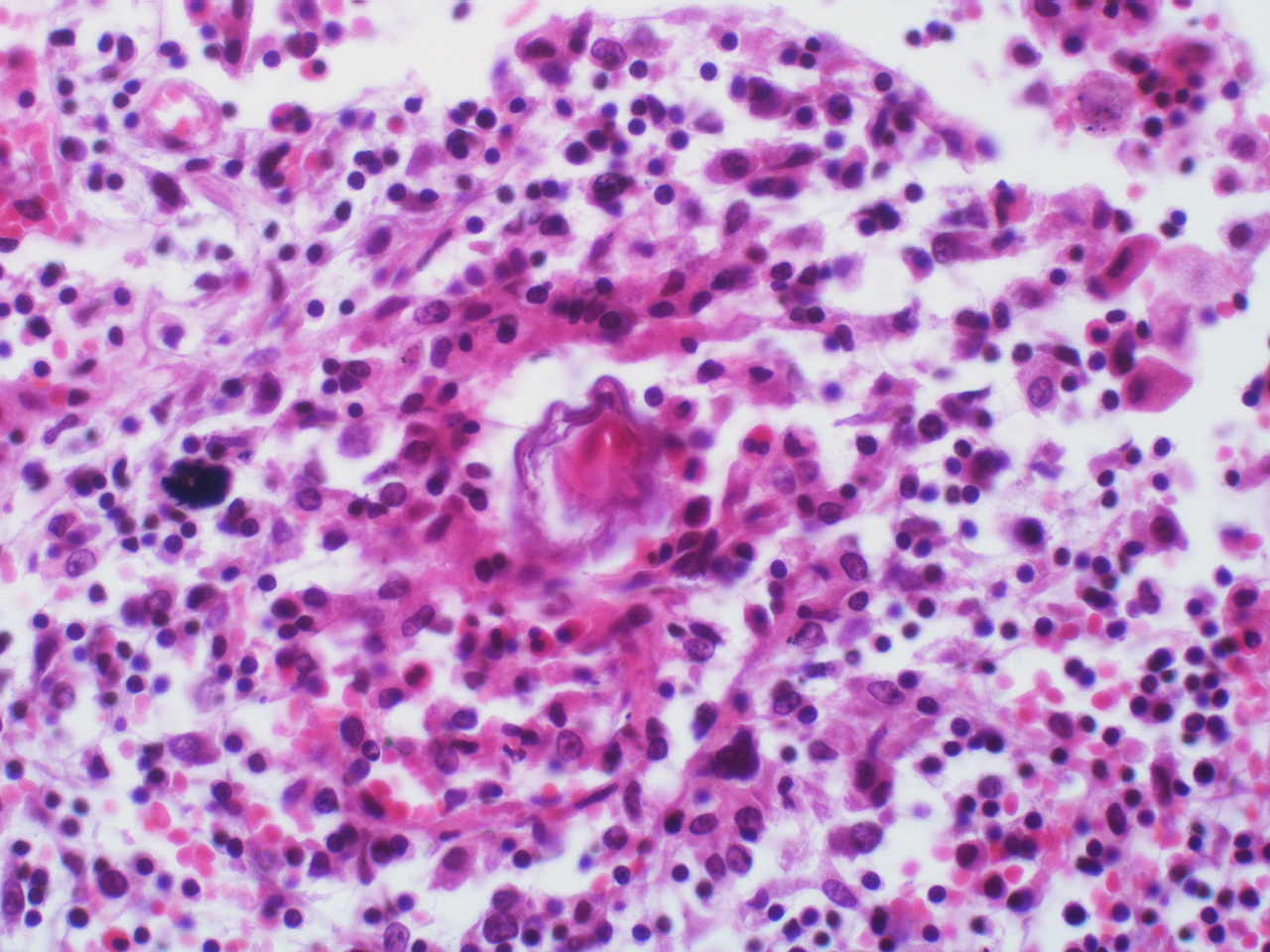
Mice and primate models have also proved invaluable in aiding our understanding of the chronic disease pathology (progression of the disease) that results from the host's immune response to accumulating tissue-trapped eggs. Secretions from these schistosome eggs cause a vigorous inflammatory response that is supposed to protect the tissues against damage from the egg toxinsANCHOR. Unfortunately, the nodule of inflammatory cells (granuloma) that accumulates around each egg ultimately leads to tissue damage due to tissue scarring and excessive pathology.
The severity of the disease seems to depend on the host’s ability to make a balanced T helper cell response (an essential part of the immune system) that is able to effectively direct granuloma development; in doing so it prevents debilitating acute disease, and minimises tissue scarring and severe morbidity during chronic infection. Data from studies in mice have established that critical control of inflammatory cytokines (secreted signalling molecules) such as Interleukin-17 and Interleukin-13 is necessary to prevent disease symptoms from becoming severeANCHOR ANCHOR.
Effects on concurrent infections
Most people who live in areas where schistosomiasis is endemic are also exposed to many other infectious diseases. There is evidence from mouse, primate, and human studies that a chronic schistosome infection may prevent an individual’s immune system from making an effective response to another infectious agentANCHOR.
Well-documented examples include schistosomiasis patients infected with hepatitis viruses; and schistosomiasis patients who show impaired immune responses to Mycobacterium bovis (the cause of tuberculosis). Tests in rhesus monkeys indicate that another critical infection, HIV-1, may be affected by schistosomiasis co-infection; more than 60% of the world’s cases of HIV/AIDs occur in sub-Saharan Africa.
It is therefore vitally important to control schistosomiasis not just because of the impact of the disease itself but also because of the potential to exacerbate other serious diseases in developing countries.
The future
Despite the global efforts of many researchers, schistosomiasis is still a major parasitic disease. Several problems remain to be resolved, such as the identification of new vaccine candidate molecules and how to deliver them effectively, and the precise mechanisms of immune regulation. We also need more knowledge of schistosome biology to appreciate how the parasites interact with, and evade, the mammalian immune system. Hopes have been raised by the recent characterisation of the transcriptomeANCHOR and genomeANCHOR of S. mansoni. With these come the potential for the discovery of new vaccine candidates and/or drug targets that will aid the fight.
Related links:
More information on schistosomiasis at http://www.who.int/tdr/diseases/schisto/default.htm
References
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6, 411
- van der Werf MJ, de Vlas SJ, Brooker S, et al (2003) Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 86, 125
- King CH, Dickman K, Tisch DJ (2005) Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365, 1561
- Wilson RA, Coulson PS, Mountford AP (1999) Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice? Immunol Lett 65, 117
- Kariuki TM, Farah IO, Yole DS et al. (2004) Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect Immun 72, 5526
- Bergquist NR, Colley DG (1998) Schistosomiasis vaccine:research to development. Parasitol Today 14, 99
- Tran MH, Pearson MS, Bethony JM et al (2006) Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med 12, 835
- Cheever AW, Hoffmann KF, Wynn TA (2000) Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today 21, 465
- Pearce EJ, MacDonald AS (2002) The immunobiology of schistosomiasis. Nat Rev Immunol 2, 499
- Wilson MS, Mentink-Kane MM, Pesce JT et al (2006) Immunopathology of schistosomiasis. Immunol Cell Biol 12 Dec 2006; [Epub ahead of print]
- Secor WE (2005) Immunology of human schistosomiasis: off the beaten path. Parasite Immunol 27, 309
- Verjovski-Almeida S, DeMarco R, Martins EA et al (2003)Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet 35, 148
- El-Sayed NM, Bartholomeu D, Ivens A, Johnston DA, LoVerde PT (2004) Advances in schistosome genomics. Trends Parasitol 20, 154
Last edited: 5 November 2014 11:31
