Bluetongue virus
Bluetongue disease is a non-contagious, insect-borne, viral disease that affects domestic and wild ruminants, mainly sheep and sometimes cattle, goats, buffalo, deer, dromedaries, llamas and antelope. Humans aren’t affected, nor are animal products or meat.
The incubation period for BTV is 5-20 days. Many animals infected with the bluetongue virus do not show signs of disease but for the others, BTV infection first manifests itself with the onset of a high fever that may last several days. The virus mainly affects small blood vessels, causing haemorrhaging, hyperaemia, and oedema in the tissue of the lips, mouth, nasal linings, and eyelids.
Swelling of the lips and tongue, accompanied by viral destruction of host blood vessels decreases the amount of oxygen that reaches the tongue tissue. Reduced oxygen delivery causes cyanosis, or the blue appearance of the tongue. The disease can, in some cases, lead to death (with a mortality rate of 2%–90%) mostly as a result of severe pulmonary oedema or bacterial complications and cause abortion or deformities in lambs or calves.
Etiology
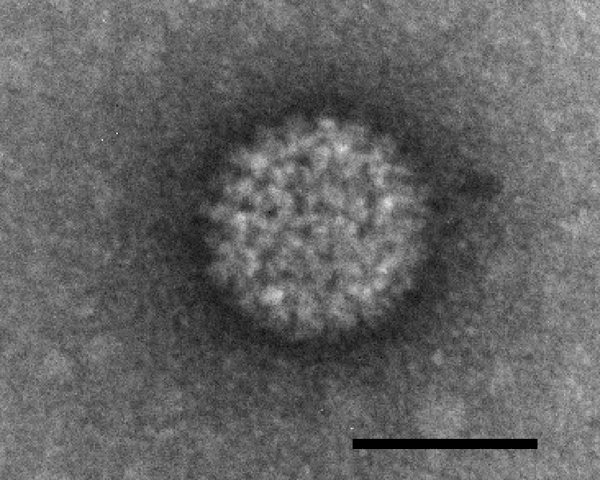
The disease is caused by the Bluetongue virus (BTV). BTV belongs to the family Reoviridae, genus Orbivirus. It is a complex non-enveloped virus with a capsid and double stranded RNA genome consisting of 10 segments of different sizes. The virus is transmitted by midges of the culicoid family and less commonly by other biting insects that become infected with BTV by imbibing blood from infected vertebrates. Bluetongue virus can persist in the blood of some animals for relatively long periods, facilitating transmission to Culicoides.
Of the over 1,400 Culicoides species worldwide, to date, fewer than 30 have been identified as actual or potential vectors of BTV. Bluetongue is not contagious and is not spread by contact between animals. However, the virus may be spread by contaminated objects (fomites), such as surgical equipment and needles. Bluetongue virus can be transferred from ruminant mother during pregnancy to the foetus during pregnancy and by infected meat consumed by dogs. The virus can be found in semen, but sexual transmission does not appear to be a major route of infection.
Although well characterized both structurally and genetically, the reproductive cycle of BTV is very poorly understood. The Reoviridae family of viruses lack an effective model for experimental manipulation, and therefore, progress in understanding the mechanisms of host cell invasion, viral replication and progeny exit in viruses such as BTV has been enormously challenging. However, it is understood that the virus is initially distributed by circulating red blood cells after transmission by blood-feeding vectors. BTV is replicated in the cells of the lungs, lymph nodes, and spleen. BTV's outer capsid proteins VP2 and VP5 are involved in cell attachment and penetration. After the host cell is infected by mechanisms still uncharacterised, BTV is uncoated, and the core particle begins a viral replication cycle and finally the progeny exit the host cell – the mechanism is again poorly understood. Researchers continue in their attempts to develop in vitro BTV genetic systems for experimental manipulation to address the numerous ambiguities of BTV replication.
Spread and reach
Although bluetongue disease was already recognised in South Africa in the early 19th century, a comprehensive description of the disease wasn’t published until the beginning of the 20th century. In 1906 Arnold Theiler showed that bluetongue was caused by a filterable agent. He also created the first bluetongue vaccine, which was developed from an attenuated BTV strain. For many decades, bluetongue was thought to be confined to Africa but, in 1943, in Cyprus, the first outbreak outside of Africa occurred was confirmed. The last outbreak in Great Britain was in 2007 and the UK government believes that there is a high risk of bluetongue type 8 spreading into the country towards the end of summer 2016, if infected midges are carried by the wind from France to the south east of England. The exact level of risk is difficult to predict because it depends on the level of disease in nearby areas of Europe, as well as the weather.
Today, infections are common across the world, only limited by the climatic and environmental conditions necessary to support the Culicoides vectors. The incidence and geographical distribution of bluetongue depends on seasonal conditions, the presence of insect vectors, and the availability of the susceptible species of animals.
The insect carriers prefer warm, moist conditions and are in their greatest numbers and most active after rain. The midge season is normally March to September, but the weather (especially temperature and wind direction) also affects how the disease can spread. Unfortunately, high affinity of the virus to blood cells contributes to prolonged viremia across the year, even in the presence of neutralising antibody. The extended viremia (up to 11 wk in cattle), and the host preference of some vectors, provide a mechanism for year-round transmission in locations where the vector-free (winter) period is relatively short.
Bluetongue virus infection has an enormous impact on sheep production. Losses result primarily from mortality, reduced production during protracted convalescence including poor wool growth, and reduced reproductive performance including temporary infertility.
Stopping the virus
To date, there is no treatment available other than supportive therapy - rest, provision of soft food, and good husbandry - but as mentioned earlier there are vaccines, but not against all the 27 different types (serotypes) of BTV which can infect domestic animals. Additionally, infection or vaccination of an animal with one serotype does not confer immunity to any of the other serotypes. Isolates differ in virulence, and some strains seem to cause few clinical signs. Like some other viruses such as influenza virus, bluetongue viruses can reassort and recombine to produce new variants. Consequently use of vaccines with different serotypes does not provide consistent cross-protection. This means that even if one serotype can be stopped; others can still be active. Vaccination needs to be precise. In many countries the timing of vaccination will depend upon local factors, in particular the occurrence of high-risk challenge periods from infected midges. In the UK the traditional mating time is from October onwards which may be later than peak Culicoides activity (late summer/autumn). Moreover, vaccines exist for just a few of the serotypes, so knowing the serotype of BTV that is causing a given outbreak is important in finding if an appropriate BT vaccine is available.
Improving diagnostics is a key area of research. Diagnosis the serotype allows for an appropriate vaccination to regions affected by the disease. The gene that encodes a virus protein 2 (VP2), located at the surface of the virus, that determines the serotype and activates immune responses, has now been sequenced for 24 serotypes, and a test has been developed to identify them (using PCR). These tests are much more rapid than conventional virus neutralisation tests, giving results within a day rather than three weeks. Research is also ongoing on novel vaccines that can provide protection against multiple BTV types.
Control of bluetongue is very difficult because of the large number of potential hosts and virus serotypes. However, containing an outbreak is possible by using a combination of quarantine and movement controls to prevent spread, by tracing and surveillance to determine the extent of virus and vector distribution, by zoning to define infected and disease-free areas and by using treatments and husbandry procedures to control vectors, reduce transmission and protect susceptible animals. It is also recommended to control of vectors by using insecticides. Protection from vectors may lower the number of Culicoides bites and subsequently the risk of exposure to BTV infection. However, these measures alone are unlikely to effectively halt a bluetongue epidemic and should be regarded as mitigation measures to be used alongside a comprehensive and vigorous vaccination program.
Last edited: 24 September 2018 13:16
