Cell, organ and tissue transplants
Moving cells around within or between different organisms so that they take another function as an individual cell, a tissue, or a whole organ.
01/03/17 Freezing organs is easy, re-warming them is not.
Transplant tissue and organs often suffer major damage when thawed out. In this new study, the researchers addressed this rewarming problem by developing a new method using silica-coated iron oxide nanoparticles dispersed throughout a solution that included the tissue.
After rewarming and testing for viability, the results showed that none of the tissues displayed signs of harm, unlike control samples rewarmed slowly over ice or those using convection heating. The researchers were also able to successfully wash away the iron oxide nanoparticles from the sample following the warming. In the new study, they warmed 50 milliliters of animal heart valves and blood vessels, indicating that the technique could be scaled up to full human organs.
http://www.telegraph.co.uk/science/2017/03/01/organ-donation-breakthrough-promised-new-tissue-warming-discovery/

16/11/16 Transplanted pig's heart survives in monkey for at least 51 days in new record
The heart was genetically modified to reduce the risk of the monkey's immune system attacking it
Muhammad Mohiuddin, a cardiac transplant surgeon at National Heart, Lung, and Blood Institute in Bethesda, US, who lead the baboon study, told Science magazine: “People used to think that this was just some wild experiment and it has no implications.
“I think now we’re all learning that xenotransplantation in humans can actually happen.”
http://www.independent.co.uk/news/science/pig-heart-monkey-51-days-survival-transplant-a7420446.html

27/09/16 Artificial blood vessel tested in lambs
Artificial blood vessels could help children with congenital heart problems and be used in heart bypass surgery. This work, tested in lambs, is promising, but needs further development before it csan be used in people.
https://www.theguardian.com/science/2016/sep/27/synthetic-blood-vessel-breakthrough-could-transform-childrens-heart-surgery
18/07/16 Scientists are developing artificial joints made of cartilage grown from our own cells.
Scientists are developing artificial joints made of cartilage grown from the cells of individuals. By using a patients' own cells, they can greatly reduce the risk of rejection. The joints also include a gene therapy to release anti-inflammatory molecules to help those with arthritis. These implants are currently undergoing animal tests to see their viability, and if all goes well they hope to test them in humans in 3-5 years.

27/06/16 3D printing of cartilage
A new 3D printing technique has allowed scientists from Pennsylvania State University to print cow cartilage so that it can be used to fix worn joints. Cartilage cannot self-repair but is a good tissue for bio-printing because it consists of only one cell type and contains no blood vessels. Researchers grew cells in thin tubes that were just three hundredths of an inch wide, made from algae, in order to produce an organic ink. A special nozzle then allowed the cartilage-ink to be pushed through to allow it to be printed in any pattern needed.
http://www.independent.co.uk/news/science/cow-cartilage-used-for-3d-printing-in-step-forward-that-could-make-patches-for-worn-joints-a7105406.html

06/06/16 Scientists create human-pig embryo to overcome worldwide shortage of transplant organs
A team of scientists from the University of California have created a technique to overcome the worldwide shortage of human organs required for transplants. Human stem cells are injected into pig embryos to produce human-pig embryos known as chimeras. They are inserted into a sow and the embryo develops into a normal pig with a human pancreas.
BBC http://www.bbc.co.uk/news/health-36437428

06/04/16 Xenotransplantation: Pig heart beats in baboon for over two years
Could organs explanted from other mammals save human lives someday? A new study shows that genetically modified pig hearts developed by US and Ludwig-Maximilians-Universitaet (LMU) in Munich researchers can survive for more than up to 2½ years when transplanted into baboons.

16/02/16 Scientists have been successfully implanting 3D-printed body parts into animals
Bone, muscle and cartilage all worked and functioned normally in the new hosts. The tissues are created with a spongelike material with micro-channels which nutrients can penetrate. When implanted into animals the plastic broke down and was replaced by natural proteins produced by the cells. Blood vessels and nerves naturally grew into the implants.
http://www.bbc.co.uk/news/health-35581454

08/02/16 Human trials with Australian designed bionic limb to begin next year after testing in sheep
Australian scientists from the Royal Melbourne hospital, the University of Melbourne and the Florey Institute of Neuroscience and Mental Health have designed a "bionic spine", a tiny 3cm long device that will enable paralysed patients to walk again by allowing them to control bionic limbs with the power of subconscious thought. The device has only been tested in sheep but human trials will begin next year in three patients at the Royal Melbourne hospital. Previous devices designed to allow paraplegics to control the movement of their exoskeleton limbs using only thought have required invasive surgery, involving removing a piece of the skull which carries a risk of infection and other complications. However, the bionic spine is minimally invasive and less cumbersome than previously developed devices.
https://www.theguardian.com/science/2016/feb/08/bionic-spine-could-enable-paralysed-patients-to-walk-using-subconscious-thought

11/01/16 human-animal hybrid embryos to grow human organs for transplantation.
Scientists have been implanting human-animal hybrid embryos into dozens of sheep and pigs in a bid to grow human organs for transplantation. By modifying the DNA of the embryos they can prevent certain organ tissues from being created, then use the human DNA to encourage a human-version of the organ to be grown inside the animal. The technology is not entirely new; for many years scientists have been humanising the immune systems of mice in order to understand diseases and test new medicines.
http://www.dailymail.co.uk/…/Human-organs-transplant-grown-…

12/11/15 60 genes modified to enable organ transplants
Researchers have modified more than 60 genes in their effort to enable organ transplants into humans.
13/11/15 blocking a specific cell signalling pathway in mice boosts ear tissue regeneration
Some amphibians and fish can regrow organs and appendages. To investigate the process in mammals, Thomas Leung, Seung Kim and their colleagues at Stanford University in California studied an engineered mouse model that is adept at regrowing injured ear tissue with no scarring.
Genes Dev. 29, 2097–2107 (2015) http://dx.doi.org/10.1101/gad.267724.115
16/10/15 Lab grown guts show promise in mice and dogs
Starting with stem cells from the small intestines of human infants and mice, scientists have for the first time grown intestinal linings on gut-shaped scaffolds that could one day treat bowel disorders like Crohn’s disease. To grow the gut lining in the lab, the researchers painted the scaffold with a sticky substance containing collagen, dribbled it with a solution of small intestine stem cells and incubated the whole. Adding connective tissue cells, immune cells and probiotics helped stem cells mature and differenciate.The tissue and scaffolding are not rejected but rather readily assimilated in lab animals. Most strikingly, the scaffold allowed dogs to heal from damage to the colon lining, restoring healthy bowel function. Other research has manage to do the same for bladders and blood vessels, among other, however, this particular technique for the intestine come closer to the shape and structure of a natural intestine than anything created before.
http://news.sciencemag.org/health/2015/10/lab-grown-guts-show-promise-mice-and-dogs

13/10/15 GM could make pig organs for humans
CRISPR, a new gene editing method has been used to manipulate pig DNA, making it a better match to human DNA in hopes of engineering safer pig organs for human transplants. The pig genome has 62 copies of porcine endogenous retroviruses which researchers were able to eliminate in pig embryos in the lab using CRISPR. The modified pig cells were unable to pass the retrovirus on to human cells and with more research it is hoped that modified animal organs will one day be used for human transplants.
http://www.bbc.co.uk/news/health-34506572
http://news.sciencemag.org/biology/2015/10/gene-editing-method-revives-hopes-transplanting-pig-organs-people

22/09/15 Lab grown kidneys work in animals
In the UK more than 6,000 people are waiting for a kidney but due to a lack in donors at least half of these will not receive a transplant and approximately 1 patient a day will die because of this. Fortunately, scientists have been able to grow kidneys in the lab using human stem cells and when transplanted into pigs and rats, they have worked, passing urine, like natural ones. Whilst more research is needed before human trials can commence, the end result of creating organs suitable for transplantation into people is a step closer.
http://www.bbc.co.uk/news/health-34312125

23/07/15 Cell transplant has the potential to completely ‘regenerate’ liver
Cell transplant has the potential to completely ‘regenerate’ liver. A study published in Nature Cell Biology showed severely damaged organs in mice could be restored to near-normal function. The main type of cell in the liver is able to restore the organ. These findings could eventually help people stuck on the waiting list for a transplant – further tests are now taking place with human tissue.
One of the researchers, Prof Stuart Forbes, said: "The big aim would be to develop a clinically applicable cell therapy for patients with severe liver failure where transplantation is not an option."
http://www.bbc.co.uk/news/health-33610569
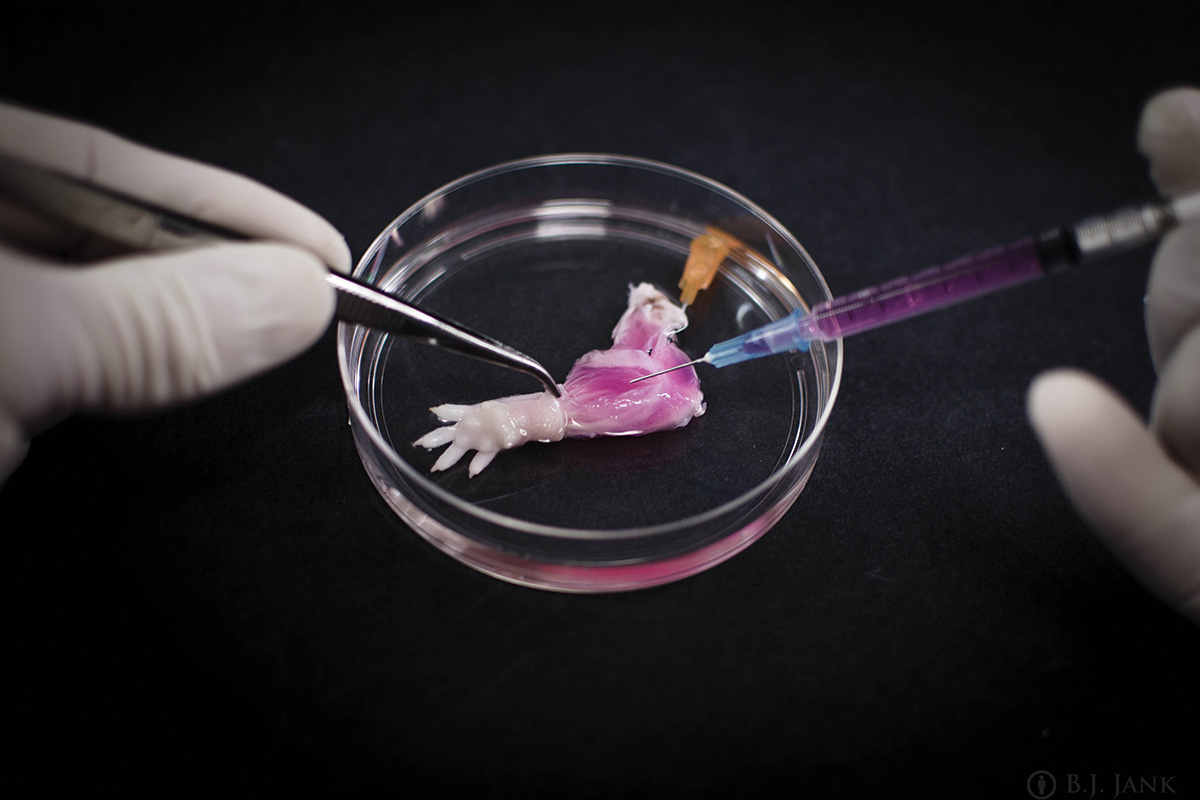
04/06/15 World's first rat forelimb grown in lab
For the first time, scientists have managed to grow a functional rat forelimb in a lab form living cells. Many amputees receive artificial replacements, but they don't function as well as real limbs. Bionic replacement limbs work well but look unnatural. Hand transplants have also been successful, but the recipient needs lifelong immunosuppressive drugs to prevent their body rejecting the hand. This biolimb offers hope that one day amputees may receive fully functional, biological replacement limbs. It gets round many of these obstacles as it only contains cells from the recipient so would avoid the need for immunosuppression and should also look and behave naturally.

08/05/15 unknown stem-cell type in mice that raises the possibility of growing human organs in large animals
Scientists stumble across unknown stem-cell type in mice that raises the possibility of growing human organs in large animals such as pigs or cows for research or therapeutic purposes. Scientists previously knew about two other types of pluripotent stem cells, but growing them in large numbers or guiding them to mature into specific types of adult cells has proven difficult. The newly discovered stem cell could be easier to grow in the lab than embryonic stem cells. These ‘region-selective’ cells grow more quickly and stably than other pluripotent cells, and therefore could be more useful for developing new therapies.
http://www.nature.com/news/scientists-stumble-across-unknown-stem-cell-type-1.17496
07/04/15 Heart muscle cells regrown in medical breakthrough, thanks to research in mice
Heart muscle cells regrown in medical breakthrough, thanks to research in mice. Unlike salamanders, humans cannot regenerate parts of their bodies when they get damaged – making heart attacks hard to recover form. But scientists have discovered a way to stimulate heart muscle cells to multiply in mice – the stimulation of a signalling system driven by the hormone neuregulin in the heart allowed the heart muscle cells to divide once again. Triggering the neuregulin pathway following a heart attack in the mice lead to replacement of lost muscle, restoring the heart.
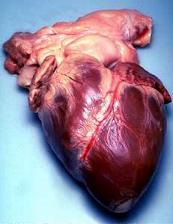
27/03/15 The first successful transplant of a non-beating donor heart was made possible thanks to animal research
The first successful transplant of a non-beating donor heart was made possible thanks to animal research. Up to now, only hearts still beating from brain dead patients were used in transplant surgeries. In the case, the heart is re-started in the donor, five minutes after death and then transferred. This new technique was made possible thanks to work done on dogs, pigs and monkeys. It has the potential of substantially increasing the number of donor hearts available for transplant by 11 to 15%.
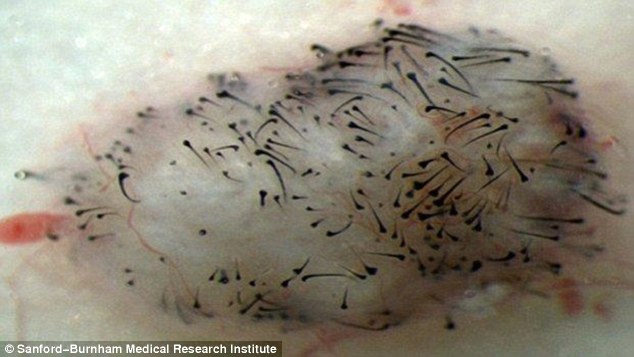
28/01/15 Human stem cells promoted growth of new hair on the leg of rats
By coaxing human stem cells to become dermal papilla cells, which are involved in follicle formation, scientists in Orlando believe they may be able to regrow human hair – and potentially a cure for baldness. Researchers managed to grow the hairs on the leg of a rat, and scientists wonder whether the technique could go on to work in humans.
http://www.dailymail.co.uk/sciencetech/article-2928737/A-cure-hair-loss-Scientists-grow-hair-rats-using-stem-cells-say-treatment-work-humans-too.html
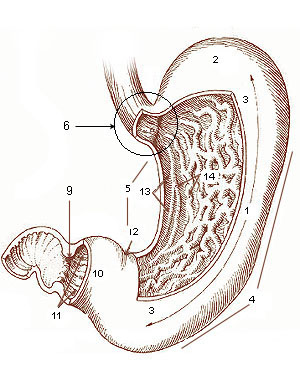
29/10/14 Miniature stomachs grown in mice using human stem cells
Miniature human stomachs, grown in vitro using human stem cells, appear to respond to infection in the same way as ordinary stomachs creating a new model in which to study human stomach disease. Many human stomach conditions are caused by infections of the bacterium Helicobacter pylori, which does not always cause disease in animal models. “There hasn’t been any good way to study human stomach disease before because animals just don’t get the same diseases,” said James Wells, director of the Pluripotent Stem Cell Facility, Cincinnati Children’s Hospital Medical Center, who led the research.
http://www.theguardian.com/science/2014/oct/29/scientists-grow-miniature-stomachs-stem-cells
21/10/14 Paralysed man walks again following nose cell transplant
A paralysed man is able to walk again following a transplant of cells from his nose, a treatment that has been pioneered in rats and dogs. Darek Fidyka, who was paralysed from the chest down in a knife attack in 2010, can now walk using a frame. Scientists from UCL developed the technique, and collaborated with surgeons in Poland to apply the treatment to Darek. Prof Geoff Raisman, chair of neural regeneration at University College London's Institute of Neurology, led the UK research team. He said what had been achieved was "more impressive than man walking on the moon".
http://www.bbc.co.uk/news/health-29645760
20/10/14 Human intestine grown in a mouse
A segment of human intestine has been grown inside laboratory mice for the first time. The fingertip-sized piece of tissue grew from a single stem cell, and was able to carry out many intestinal functions. As well as providing further evidence that whole organs could be grown from scratch inside a patient’s body, this technology could also speed up the development of new medicines by producing better laboratory models, and offer alternatives to using animals. This study “provides a new way to study the many diseases and conditions that can cause intestinal failure, from genetic disorders appearing at birth to conditions that strike later in life, such as cancer and Crohn’s disease,” Dr Michael Helmrath, who led the research, said.
Last edited: 27 September 2017 17:29
