Human papillomavirus (HPV)
Human papillomavirus (HPV)
Papillomaviruses are a diverse family of common non-enveloped DNA viruses that infect different host species. Several hundred species or types of papillomaviruses have already been identified in mammals but also other vertebrates such as birds, snakes and other reptiles, turtles and fish. However, Papillomaviruses are usually considered as highly host- and tissue-tropic, and are thought to rarely be transmitted between species.
They cause a range of symptoms, from barely detectible ones to small benign tumours, known as papillomas or warts. However, some types of papillomaviruses will be much more dangerous and cause cancer. Cervical cancer but also anal cancer, penile cancer, vulval cancer, vaginal cancer, and some types of head and neck cancer in humans can be linked to human papillomavirus (HPV) infections (mostly HPV-16 and HPV-18). There are over 100 different types of HPVs including 30 that are sexually transmitted. (1)
HPV is the second most common sexually transmitted infection in the UK, after chlamydia, and is the most common sexually transmitted infection globally. More than 90 percent of sexually active men and 80 percent of sexually active women will be infected with HPV in their lifetime. Most will not experience any symptoms, but some will go on to develop cervical cancer that kills more women worldwide than any other type of cancer apart from breast cancer. Luckily, there is now a vaccine that protects against the most aggressive types of HPVs.
Discovery of Papillomaviruses
Papilloma viruses were first identified in the early 20th century, when it became clear that these skin warts, or papillomas, could be transmitted between individuals by a filterable infectious agent.
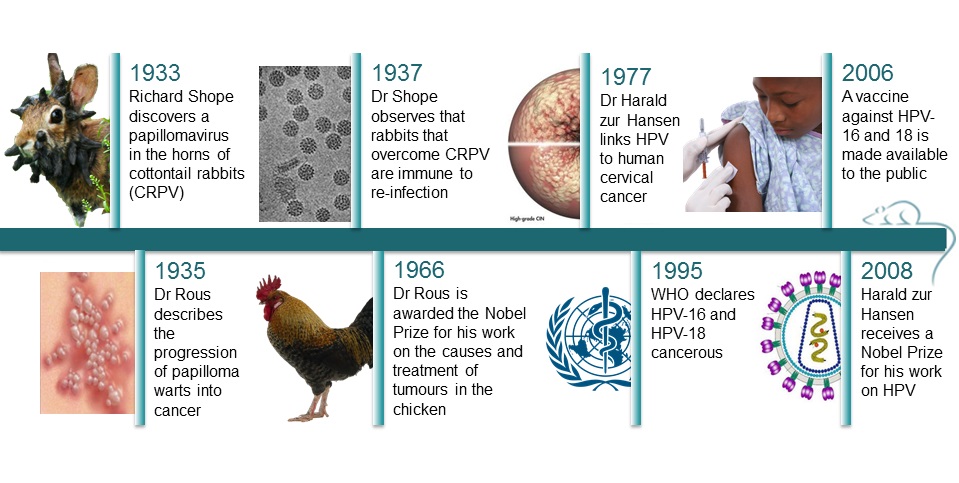
In 1933, rabbits with horny growths on their head caught the eyes of a scientist called Richard Shope. After catching them, removing and grinding their horns, filtering the mixture to recover particles smaller than cells, and re-inject those particles into other rabbits, he found that the injected rabbits started growing horns too, hinting that viral particles might be responsible for the outgrowths. The horns were in fact warts, and Shope’s work led to the discovery of the cottontail rabbit papillomavirus (CRPV), a cancer-causing virus. (15)
Animal and human papillomaviruses have a lot in common and the cottontail rabbit was the first animal model for cancer caused by a virus – the cottontail rabbit papillomavirus (CRPV). It helped enormously in the development of vaccines against cervical cancer, especially because the Human Papillomavirus (HPV) could not be replicated in cell culture, nor could it be transmitted to other mammals. (1)
The work was furthered by Dr F. Peyton Rous, who showed in 1935 that a papillomavirus could cause skin cancer in infected rabbits. This was the first demonstration that a virus could cause cancer in mammals. Rous showed that these benign tumours could become malignant following exposure to certain chemicals. He proposed that the transformation of normal cells into cancerous cells may consist of several stages, such as virus-exposure, hormonal changes or exposure to chemical agents. For his work, he was awarded the 1966 Nobel Prize for Medicine.
Dr F. Peyton Rous had previously described in 1910 the existence of a cancer-causing sarcoma virus in chickens. He had filtered cells from some malignant connective tissue that had appeared spontaneously in a chicken and injected the mixture into healthy chickens, which then developed similar tumours. His experiments showed that the filtrate contained the cancer-causing agent that eventually became known as the Rous sarcoma virus. Although its significance was not clear at the time, this was the first virus known to transform normal cells into cancer cells.
Cancer causing agents
It took a while before scientists fully accepted that a virus could be responsible for cancer. It wasn’t until 1995 that the World Health Organization (WHO) declared that HPV-16 and 18 were cancerous.
In 2002, Dr Saverio Campo from Glasgow University describes “ a paradigm shift in the late 1970s for some viruses to be recognized as ‘tumour viruses’ in humans”. (16)
Indeed, in 1977, Dr Harald zur Hansen published the first research linking the papillomavirus directly to cervical cancer. This was confirmed in the early 1980s when he isolated two previously unknown types of HPVs – HPV16 and 18 - from cancerous tissues. The results were undeniable, and he was awarded the 2008 Nobel Prize of medicine for his work. Researcher later found that those two HPV types, specifically, are responsible for 70% of cervical cancers in women (17), the remaining can be caused by 13 other types of HPV. Today we know that HPV is responsible for over 80% of cervical cancers in women and 70% of throat cancers in men. (2)
The reason why it took so much time to confirm that viruses can cause cancer is essentially because viruses are necessary but not sufficient to cause cervical cancer. Most HPV infections do not result in cancer - most are cleared by the immune system or are simply not dangerous. More than half of sexually active women are infected with HPV but only a small percentage go on to develop cancer.(1) The virus needs an extra ‘push’ to cause cancer. For example, Bovine papillomavirus (BPV) only causes cancer in cattle that eat bracken. Risk factors such as smoking, genetic predisposition, or even side effects from the body’s defence mechanisms could trigger cancers in humans. (9)
Animal research to understand HPV pathogenesis
Due to the species specificity of papillomaviruses, infection of experimental animals with human papillomavirus (HPV) is not possible. However, understanding the natural history and carcinogenic potential of HPVs is assisted by the study of several animal papillomaviruses.
Animal papillomaviruses have been studied both as models of human papillomavirus infection and as agents of disease in animals. For many years now, bovine and cotton tail rabbit papillomaviruses have been the model systems of choice to study the behaviour of HPV. Induction of papillomas and their neoplastic progression has been experimentally demonstrated and reproduced in cattle and rabbits, and virus-cofactor interactions have been elucidated in these systems.
In addition to the study of papillomavirus infection in whole animals, in vitro studies with animal papillomavirus proteins have contributed greatly to the understanding of the mechanisms of cell transformation. In vitro studies have notably contributed to the elucidation of viral gene control and cell transformation that were later confirmed in cattle and human cell models. With the advancements in molecular and cell culture techniques, the direct study of HPV has become less problematic and understandably research efforts have shifted in focus from animal to human papillomaviruses. However, there are still areas in which studies on animal papillomaviruses will continue to provide answers to questions pertaining to virus biology.
Similarities between animal and human papillomaviruses are such that the study of one is still very much important to understand the other. For example, the same involvement of HPV in oesophageal and bladder cancer in humans can be seen in cattle with BPV. Equally, both BPV and HPV seem so stay in latency in lymphocytes, and only experiments performed in animals could clarify this point.
Together, in vitro and in vivo studies have shown how the virus inserts its own genetic code into an infected cell and either destroys it to release viral particles or transforms it into a cancer cell giving it viral properties such as unlimited growth
Animal papilloma viruses
Animal papillomaviruses cause distressing diseases in both farm, companion and wild animals. It can cause conditions such as teat papillomatosis in cattle, equine sarcoidosis and canine oral papillomatosis. In parallel to the need to understand the human infecting papillomaviruse, there is also some urgency in identifying the pathogenesis of these problematic infections in animals and finding cures. Indeed, persistent and florid teat papillomatosis in cows can lead to mastitis, preventing the suckling of calves and making milking impossible. Equine sarcoidosis are often recurrent and untreatable and lead to loss of valuable animals. Canine oral papillomatosis can be very extensive and persistent and lead to great distress. The continuing research in the biology of animal papillomaviruses is amply required.
Non human primate papillomavirus
Many different papillomaviruses were isolated from different species of non-human primates, including the colobus monkey (CgPV), the pygmy chimpanzee (PCPV, evolutionarily related to HPV-13), the howler monkey, cynomolgus macaques and rhesus monkeys (RhPV).
Bovine papillomavirus (BPV)
BPVs are a heterogeneous group of viruses that are distributed worldwide. They induce papillomatosis of the skin, the genital and paragenital area, the eye, the upper gastrointestinal tract and the urinary bladder. More than 20 different types exist. BPV can be transmitted from infected females to susceptible calves through skin contact during suckling or from bulls to females during breeding.
BPV can infect non-human primates, hamsters, mice (transgenic and xenografts), and equids – horses and donkeys - among others.
Cottontail rabbit Papillomavirus (CRPV)
The discovery of the CRPV and its link to cancer developments more specifically to carcinomas constitute hallmarks in the history of viral oncology. A number of important properties of papillomaviruses such as the role of certain viral genes in the development of papillomas and carcinomas and the synergism between virus and chemical co-carcinogens were established for the first time using CRPV.
Rabbit papillomaviruses are endemic in certain parts of the USA. In nature, CRPV infects primarily cottontail rabbits and occasionally jackrabbits. However, infectious virus has been obtained from papillomas induced in jackrabbits and snowshoe hares (Lepus americanus) and the host range has even been extended to rats under experimental conditions.
Domestic and commercial rabbits have their own papillomavirus (ROPV) which was studied in mice and hamsters.
Mouse papillomavirus (MnPV)
The only known MmPV was isolated from a zoological colony of European harvest mice. MmPV could be transmitted to one of two harvest mice but not to laboratory mice (CAF or C3H strains) or to wild deer mice (Peromyscus maniculatus gambeli).
Equine Papillomavirus (EqPV)
Equids can also develop genital, cutaneous, ocular, and oral papillomas and squamous-cell carcinomas. Research suggests the existence of at least two different types of EqPV.
The papillomavirus in horses is very similar to the human virus, which means that similar vaccines were developed – the technologies were transferable from one species to the next. (12, 13)
Papillomavirus in Cervidae
Papillomavirus DNA was isolated from cancers of different members of the cervidae family, such as European elk(EEPV or EPV), reindeer (RPV), red deer (RDPV), mule deer and white-tailed deer (DPV). The genomes of EEPV or RPV induced cancer in Syrian hamsters.
Ovine papillomatosis (OVP
Ovine papillomatosis, that affect goats and sheep, is caused by ovine papilloma virus but it is uncommon when compared with other animal species.
Mastomys natalensis papillomavirus (MnPV)
Mastomys natalensis is a common rodent in southern Africa. Interest in these animals was stimulated by the early observation of Oettlé (1957) that 28–53% of Mastomys that were older than 1 year suffered from stomach cancer. As the incidence of stomach cancer is extremely low in other laboratory rodents and was highly variable in the different Mastomys laboratory colonies, exogenous causal factors were postulated. MnPV DNA was found in various tissues from animals of such colonies, whereas colonies that did not display a high rate of spontaneous stomach tumour formation were free from MnPV.
Canine oral papillomavirus (COPV)
Dogs can be affected by oral papillomatosis, particularly if kept in kennels in large numbers. Progression to squamous cancer is rare, but has been observed. A vaccine against the virus exists.
Feline papillomas
The Felis domesticus papillomavirus was cloned from hyperkeratotic cutaneous lesions of a Persian domestic cat and sequenced. Cats tend to display clinical papillomaviral lesions when immunosuppressed either by FIV infection or by steroid therapy.
Avian Papillomavirus
The DNA of a Fringilla papillomavirus (FPV) was isolated from cutaneous papillomas in chaffinches but could not be transmitted to other chaffinches, canaries or hamsters. an avian papillomavirus genome was also cloned from a cutaneous papilloma from an African grey parrot (PePV).
Papillomavirus in wildlife
Papillomaviruses occur also in wild animals, mostly in mammals. Studies have tried to map the spread of wildlife papillomavirus (WPV), often with a bias toward the Northern Hemisphere, which may have influenced results to show Europe as the most likely origin of the available WPV lineages. The study of clinical and subclinical occurrence, distribution, and phylogenetic signatures of WPV may help understand the spread, virulence, and epidemiology of papillomaviruses in general. WPV are a promising host-pathogen system to untangle questions regarding co-evolution due to its large geographic distribution and occurrence in a wide variety of aquatic and terrestrial wildlife species.
Papillomas in Dolphins
Dolphins get multiple infections of papillomaviruses. There are also the only species besides humans known to harbour co-infections, or infections of multiple papillomavirus types in the genital mucosa. Up to 8 HPVs have been reported in humans at the same time. Co-infection is believed to be one of the biggest risk factors for the development of cervical cancer in humans.
Dolphins are also exceptional in that they don’t develop the disease even when infected by the virus. If we can figure out why, the information might be applied to preventive strategies for humans.” (10, 11)
Finding a vaccine
As with most medical discoveries, animal research and the study of animal papillomaviruses played a vital role in the development of the HPV vaccine. Cattle, rabbits, mice, non-human primates, dogs, and more, all provided the opportunity to study vaccination in the natural host.
The story starts in 1937. Shope noticed that the rabbits that overcame a CRPV infection were immune to reinfection. Using CRPV in rabbits, BPV in cattle and canine oral papillomavirus, researchers found that whichever the animal, it was possible to protect against the papillomavirus. Thus it was possible to stop the development of the papillomas or the cancers through various methods of immunization.
Creating a vaccine was problematic because even the DNA from deactivated viruses can cause cancer. Additionally, each papillomavirus can only be cultured in its respective host species. It wasn’t until the early 1990s that scientists were able to determine what the components of a vaccine should be : virus-like particles (VLPs), multiple copies of the main structural protein of HPV.
Injection of bovine papillomavirus capsid protein L1, a protein that forms the outer shell of the virus, was found to induce a strong immune response in rabbits, and that the rabbits produced antibodies that bound strongly to bovine papillomavirus in vitro. Researchers now needed to determine if delivering the VLPs were safe and effective protecting against HPV. Because of the species-specificity of the papilloma viruses, animal efficacy trials had to be done with the animal equivalent of the vaccine. Species-specific vaccines were created for rabbits, cows and dogs using the corresponding viral outer shell, and all promoted high levels of antibodies and were 90% effective at preventing warts following exposure to papillomavirus. (18)
Eventually John Kneider came up with a way to grow human papilloma virus (HPV) in nude mice. These mice have a defective immune system that does not reject tissue transplants from other animals so Kneider transplanted human cervical tissue and foreskin tissue onto the mice, which were then used to test for a vaccine. (3) VLPs of human papillomaviruses were tested in nonhuman primates to see if they could induce an immune response, and they did.
Clinical trials with human volunteers showed that the HPV VLP vaccines induced high levels of antibodies against HPV. Women vaccinated in the trials were also protected from persistent HPV infection and precancerous cervical changes. Because of the success in the human trials, Gardasil, the first vaccine against HPV, was approved by the FDA in 2006. The vaccine, Gardasil, protected at the time against four different HPV types: the two most prevalent high-risk viruses, HPV16 and HPV18, and the two most common causes of benign genital warts, HPV6 and HPV11.
Effective vaccination campaigns have since limited the spread of HPV infection. In the UK, a school-based HPV vaccination programme for 12–13-year-old girls began in 2008 and in boys in 2019. The prevalence of HPV 16/18 infection among these youngest women decreased from 13.5% in 2014 to 3.1% in 2018 along with a halving of high-grade cervical intraepithelial neoplasia. In the US, vaccination against human papilloma virus resulted in a 64% reduction in infections in girls aged 14-19 .
However, HPV-16 and 18 only account for 70% of the HPV linked cervical cancers. 13 other HPVs remained unstoppable by the first Gardasil vaccine. The vaccine needed to affect a broader spectrum of the virus. In 2015, FDA approved a new version of the vaccine that is effective against nine types of HPV. Gardasil 9 protects against HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58. Between them, types 16 and 18 are the cause of most cervical cancers in the UK (more than 80%). Types 31, 33, 45, 52 and 58 cause an additional 15% of cervical cancers.
Protection against HPV is particularly important to human health given that these viruses are responsible for almost all of cervical cancers in women – one of the most deadliest in women. The effectiveness of the HPV vaccine is excellent news in our quest to reduce the deadly toll of cervical cancer. The new vaccine, coupled with a boost in cervical cancer screening could save up to 10 million pounds each year to the UK NHS. (8)
References
- https://www.animalresearch.info/en/medical-advances/diseases-research/cervical-cancer/
- http://www.understandinganimalresearch.org.uk/news/research-medical-benefits/sex-virus-blamed-for-cancer-rise/
- http://faseb.org/portals/2/pdfs/opa/2008/HPV.pdf
- http://www.cdc.gov/media/releases/2013/p0619-hpv-vaccinations.html
- http://www.sciencesetavenir.fr/sante/20150121.OBS0460/cancer-du-col-de-l-uterus-40-des-femmes-negligent-le-frottis.html
- http://www.cancerresearchuk.org/about-us/cancer-news/news-report/2014-10-07-experimental-hpv-vaccine-could-boost-cervical-cancer-protection-worldwide
- http://scienceblog.cancerresearchuk.org/2014/09/16/hpv-the-whole-story-warts-and-all
- http://www.cancerresearchuk.org/about-us/cancer-news/news-report/2014-06-09-cervical-screening-boost-would-save-ps10m
- http://www.cancerresearchuk.org/about-us/cancer-news/news-report/2014-06-06-immune-system-friendly-fire-triggers-hpv-linked-cancer
- http://news.ufl.edu/archive/2010/02/dolphins-could-be-ideal-model-to-study-human-cervical-cancer-uf-veterinarians-say.html
- http://www.thehindu.com/sci-tech/health/dolphins-help-scientists-understand-cervical-cancer/article111947.ece
- http://www.understandinganimalresearch.org.uk/news/research-medical-benefits/horse-genital-cancer-virus-identified/
- http://www.ncbi.nlm.nih.gov/pubmed/21039805
- MRC National Institute for Medical Research, A century of science for health
- Shope RE (1933) Infectious papillomatosis in rabbits Journal of Experimental Medicine 58 607
Last edited: 27 January 2023 11:12