Blood transfusion
Animals and blood transfusion
The storage and transfusion of sterile, compatible blood, blood constituents or blood substitutes is a routine and life-saving procedure. Animal experiments were crucial to the development of a) the concept of the benefit of blood transfusion, b) techniques for carrying out transfusion and c) the preservation of blood and thus to the establishment of blood banks.
Nowadays, over 118.5 million blood donations are collected each year globally – enough to fill 43 Olympic-sized swimming pools. However, this is still not enough to meet demand. Research is ongoing to find alternative sources of blood and even alternatives to blood.
Understanding blood circulation
In 1657, William Harvey studied and mapped out heart function and the circulatory details of the blood through arteries and veins. Harvey used about 50 species to elucidate the nature of circulation.
He studied, by means of a magnifying glass, the motion of the heart in a small shrimp found in the Thames. Using ligatures on the slowly beating hearts of cold-blooded animals, he then went on to show that the heart pumped blood via a closed circulation through arteries to the veins. Experiments on sheep, deer and dog further demonstrated that the heart pumped in unit time a larger quantity of blood than can found be in the whole body – thus clearly demonstrating a circulatory system.
By the early 17th century, the concept that blood was pumped via the arteries to the organs was well established. Blood began to be perceived as a transport system. Experiments in living animals started testing this system as a conveyor of medicine.
Early transfusion experiments
In 1657 Christopher Wren, better known as the architect of St Paul’s Cathedral in London, became the first person to conduct an intravenous injection. Accompanied by Robert Boyle, he successfully injected, via an inserted quill attached to a bladder (in later experiments, a syringe) into a superficial vein of a dog, first wine, ale, opium, emetic, and antimony oxide into a dog’s veins. The success of the injection was judged by the dog’s intoxication.2
This experiment led to attempts to transfuse blood from one animal to another. The earliest, well-authenticated account of the transfusion of blood from one dog to a second is that of Richard Lower, who in February 1665 used quills (and later a silver tube) to transfer blood from the carotid artery of one dog to the jugular vein of a second (see figure 1).
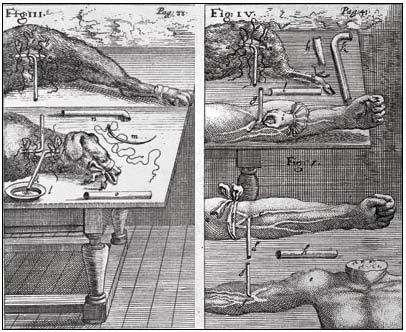
Fig 1. Engraving showing transfusion in the neck and leg of a dog, from animal to man, and from man to man, by J. S. Elsholtz, 1667. Wellcome Library, London, CC BY.
The first person to transfer animal blood to a human was the French philosopher and mathematician Denis. With the help of the surgeon Emmerez, Denis allegedly transferred 9 ounces of blood from the carotid artery of a lamb into a 15 year old child in 1666. (see fig 2) Apparently, astonishing improvement resulted. Denis performed the operation on a further three subjects with no untoward effect.
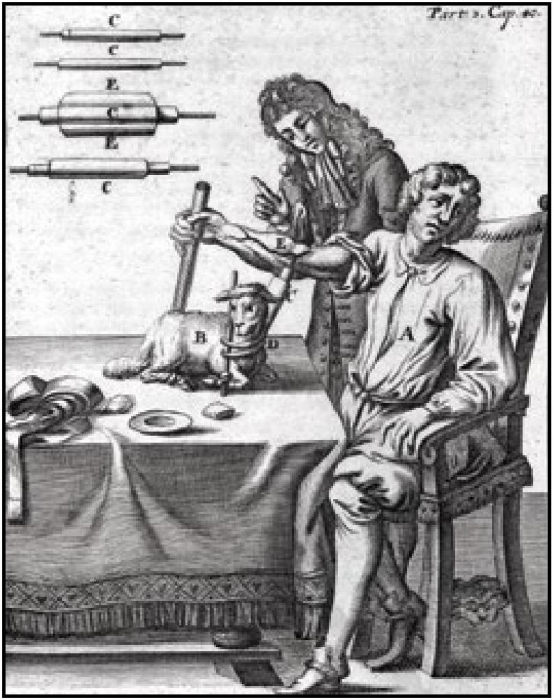
Fig. 12.2 Attempt at blood transfusion from lamb to man, depicted in an illustration dating from 1705. Wellcome Library, London, CC BY.
Unfortunately, Denis’ last patient was a man presumably suffering from neurosyphilis, who died after 3 transfusions with calf’s blood . Denis had enemies prominent in the Faculty of Medicine of Paris who were implacably opposed to transfusion of animal blood into man. He was charged with murder but eventually exonerated after counter charges that the patient had been poisoned by his wife. Nevertheless experiments on transfusion of blood into humans were prohibited by an edict of the French Parliament.
Delayed discoveries
This official ban on transfusion in France in the early 18th century and the opposition to animal experiments “delayed the practical availability of blood transfusions and led directly to the deaths of patients” according to scientists. If transfusions between different species had been investigated before the transfusion of animal blood to man, the incompatibility of bloods of different animal species would have been established in the 17th century instead of 150 years later.
During the 18th century, references to transfusion were rare, and those described were irrational (in 1792 for example, Russell claimed to cure a child of rabies by injection of lamb’s blood). At the time transfusion was a technically difficult procedure - the only available apparatus were animal quills or silver tubes - and there was no way to prevent blood from clotting. Despite this, the practice continued as a niche procedure and new intravenous solutions were used including saline, milk and albumin extracted from egg.4
The gradual accumulation of physiological and pathological knowledge changed the perspective with which transfusion was viewed. Rosa and Scarpa (1788) recommended transfusion as a treatment for anaemia, and in 1796, Erasmus Darwin (grandfather of Charles) advocated transfusion of blood in cancer of the oesophagus and other conditions resulting in inadequate nutrition.
The first documented successful human to human transfusion did not happen until 1830, when James Blundell transfused blood from his assistant to a woman suffering haemorrhaging after birth.
The first human transfusion for haemorrhage
This first transfusion was only made possible thanks to previous work in animals. In 1821, Provost and Dumas showed that animals that had hemorrhaged to the point of death could be revived by blood transfusion, but not serum or water warmed to 38°C. Blood from animals of other species was not effective, since the animals appeared to survive but succumbed within a few days.
Provost and Dumas did not attempt transfusion in humans. Blood transfusion in humans was established as a sound scientific and clinical procedure by James Blundell (1790-1877), a lecturer in physiology and midwifery at the United Hospitals of St Thomas and Guy. Blundell was moved by the many deaths he had witnessed in patients with post partum haemorrhage. Even when bleeding had been suspended, frequently the patients had lost so much blood that one could do nothing but observe them sinking until death followed within 2-4 hours.
He had also previously done experiments in dogs that established that death from haemorrhage could be prevented by transfusion of blood from the same species, even if vital signs had been lost. Recovery occurred even if the volume of the blood transfused was a fraction of that lost (even just 20%). Transfusion of blood from another species was not effective, but venous blood was as effective as arterial blood, even if its transfusion was delayed or if it was passed through a syringe.
After his experiments in animals, Blundell took the giant step of attempting transfusion of human blood to patients with severe haemorrhage. He performed the operation eleven times, at first only as a last resort in patients who were clearly irrecoverable. A typical case was reported in The Lancet in 1828 . In view of the obvious difficulties associated with supplying blood by the direct connection of the donor’s artery to the recipient’s vein, Blundell developed apparatus that obviated the need to cannulate the vessel of the donor. Venous blood was allowed to collect into reservoirs from whence it was pumped by syringe or allowed to flow under gravity (Blundell’s “impellor” and “gravitator”; Fig. 3 & Fig. 4).
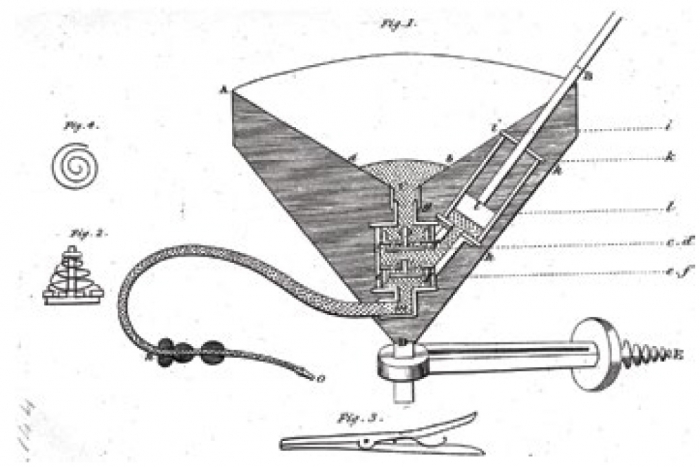
Fig. 3 Drawing of Blundell’s impellor, which allowed venous blood to be collected in reservoirs before being pumped or allowed to flow under gravity to the recipient. (From Ref. 9). Wellcome Library, London, CC BY.
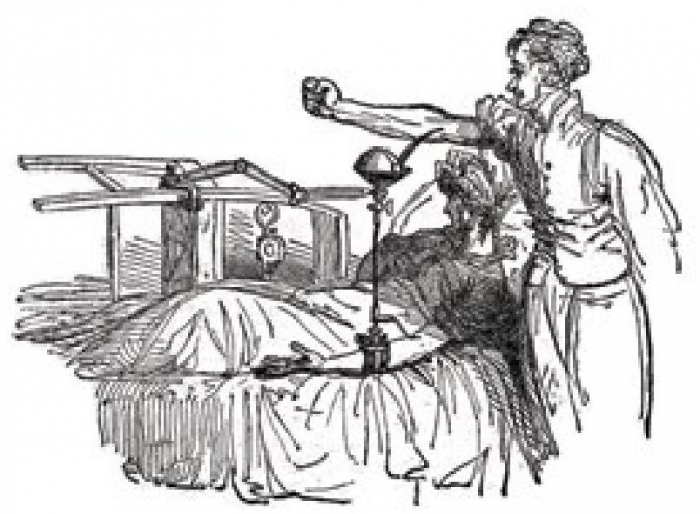
Fig. 4 Blundell’s apparatus in use. From J. Blundell (1828). ‘Observations on the transfusion of blood’, The Lancet, 2, 321. Wellcome Library, London, CC BY.
Blood transfusion was thus established as a respectable and valuable procedure. Used as a last resort only, it was not however commonly practiced. The procedure remained quite difficult to carry out, and if reasonable volumes needed to be transfused, the clotting that was likely to occur meant that one had to transfuse by cannulation and connection of the artery of the donor to the vein of the recipient. It wasn’t easy to persuade donors to have an artery cannulated and it was hard to regulate the amount transfused.
The ABO Blood group system
Blundell, and previous researchers emphasised quite importantly the need to transfuse blood from individuals from the species. In 1869, Crile showed the first evidence as to why. Serum from different animals caused human red blood cells to clump. Landois further explained the phenomenon by showing that animal serum caused actual lysis of human blood cells, much like it would act against bacteria, thus explaining post transfusion haemoglobinaemia and the excretion of black urine in some early transfusions. These experiments definitively established that transfusion of animal blood to humans was absolutely prohibited pending health complications.
Immunological studies provided clues to the reason for the incompatibility of human red cells with animal sera. Bordet found that the red cells of some species could generate antibodies in the plasma of another. Prompted by earlier work, particularly that of Landois, Landsteiner was intrigued by the biochemical species specificity and considered whether it could somehow be also transferred at the individual level, within the same species. Mixing the serum and red cells of different human individuals, Landsteiner discovered that humans were divided into different blood groups, which meant that recipients and donors could be matched. He eventually established the ABO blood groups system, which greatly improved the reliability of transfusions. This obviously had great significance for the transfusion of compatible blood. Landsteiner’s work explained the failure of some early transfusions, however it was still an impractical technique because blood coagulated quickly.
Some transfusion reactions occurred even with matched samples in the ABO system. In the 1920s, Landsteiner and Levine detected other agglutinins (MN and P) in all four blood groups by injecting rabbits with human blood and showing the presence of raised antibodies to the human antigens.
Transfusion methods
Even though the matching of blood reduced transfusion reactions, at the start of the century the problem of clotting still prevented blood transfusion becoming routine. Blood clots, in contact with anything other than the endothelial surface, would potentially form. If this happened in the transfusion cannulae, they would then enter the circulation, causing pulmonary embolism. For this reason, transfusion was rarely performed, particularly when infusions of isotonic salt solutions became popular.
Bypassing cannulae completely, Carrel developed in 1902 a technique in animals for joining arteries to veins, thus providing a continuous endothelial surface that enabled blood to flow from one vessel to the other without clot formation. This technique was put to practical use in a celebrated case that occurred a few years later, involving a 5 day old infant.
Carrel’s technique was not simple, but Crile perfected it in 1907 based on 225 experiments in animals and 32 clinical cases. He developed an easier method using a carefully constructed ring through which a vessel could be pushed and then everted over.
Anticoagulants & blood storage
By the early 1900s, the prevention of blood coagulation was intensively investigated. Some delay in clotting was achieved by using paraffin-wax coated vessels. Some experimenters toyed with the use of the anticoagulant substance extracted from leeches, hirudin. Lewisohn tested hirudin in dogs, one of which died. After inconclusive tests in humans, hirudin was removed as a candidate anticoagulant. The breakthrough came from Adolph Hustin in 1914, who found that adding sodium citrate to blood prevented it from clotting, and that the citrated blood could be safely transfused into dogs.
It is difficult to assign who first advocated the use of citrate in blood. The contenders are Hustin, Agote, Weil and Lewisohn. Most accounts accept that the Belgian, Hustin was the first to infuse citrated blood to a patient. However, Lewisohn probably deserves the most credit since his careful experiments established the minimum concentration necessary to prevent clotting, and the amount of citrate likely to produce a toxic effect with a series of animal experiments. In 1915 Richard Lewisohn advanced this finding by determining the maximum amount of citrate that could be transfused into dogs without toxicity. This revealed the optimum concentration of sodium citrate that could be added to blood for the best anticoagulant effect.
A further break-through came in 1915 when Richard Weil8 showed that this citrated blood could be stored for two days and still be effective when transfused into guinea-pigs and dogs that had lost blood.
These experiments were followed by Peyton Rous and Joseph Turner’s work on rabbits in 1916,9 demonstrating that with certain additives and proper treatment, blood could be stored for 14 days and then successfully transfused. However, animal experiments alerted physicians to the possible danger of transfusing large volumes of blood that had been stored for a long period. Infusions in rabbits showed that whereas the citrate or potassium in transfused blood produced only mild toxicity, a combination of both killed 15 out of a group of 19 rabbits. Thus the toxic effects of citrate and potassium reinforced one another.
All of these experiments made the prolonged storage of blood possible, enabling the establishment of blood banks and blood transfusion to become a routine procedure. The first recognised blood bank was set up in the 1930s at the Central Institute of Haematology and Blood Transfusion in Moscow. By 1937, 6,000 effective transfusions of stored blood had been made. Peyton Rous was awarded the Nobel Prize in 1966, for his many contributions to medicine including the discovery of cancer-causing viruses.
Today, the storage and transfusion of sterile, compatible blood or blood constituents is a routine and life-saving procedure.
- The anticoagulant Heparin
The potent anticoagulant heparin is not widely used to prevent the coagulation of blood intended for transfusions. It is, however, used for venous thrombosis and to prevent blood clotting during open heart surgery and kidney dialysis.
Heparin was discovered serendipitously in the liver of dogs during a search for endogenous clotting substances. Heparin for clinical use is obtained from pig intestine or bovine lung, and the crude extract is standardised by measurement of its anticoagulant action on sheep plasma (BP and USP
The Rhesus Factor
It is difficult to establish who first discovered the Rhesus (Rh) antigen. A reasonable interpretation is that the Rh antigen was discovered in Landsteiner’s laboratory in the early 1930s, by injection of rhesus monkey red cells into rabbits and guinea pigs, and adding their plasmas (containing anti-Rh antibodies) to human red cells. Landsteiner, however did not publish the work until 1940.
Meanwhile Levine (a former collaborator of Landsteiner) together with Stetson, after work in rabbits, discovered in 1939 an antibody (later shown to be anti-Rh) in the plasma of a woman whose child had died in utero.
Artificial blood
In 2011, a blood substitute derived from cow plasma was used to save the life of a woman with only 1 litre of blood left in her body but whose religion forbade conventional blood transfusion.10 The haemoglobin-based oxygen carrier used in this case (Hemopure) does not require matching blood types and can last without refrigeration for up to three years.
This is one type of so-called artificial bloods that are designed to increase oxygen transport in the body after heavy blood loss. However, these have had a controversial history.
A meta-analysis of studies suggested a 30% increased risk of death from the use of artificial blood.11 The FDA drew criticism from the US Navy, who had funded much of the research, for stopping clinical trials and the author of the meta-analysis was sued by one of the companies who developed the treatment.12-13 Meanwhile, after being initially approved for use in South Africa, it was later withdrawn following safety concerns.14
Growing red blood cells
Researchers have also been working towards creating a continuous supply of red blood cells from embryonic stem cells. After first developing the technique in mice, researchers in Japan developed an immortalised cell line of human embryonic stem cells that could be used indefinitely to produce red blood cells.15-16 171819
Work in this field has been delayed because of ethical concerns and licensing for using cells derived from human embryos, but despite these reservations clinical trials were started . In 2022, the first ever clinical trial transfused laboratory grown red-blood cells into people.
In 2011, red blood cells were grown from haematopoietic stem cells extracted from bone marrow.20 These were cultured to produce 10 billion cells (the equivalent of 2 ml of blood) and, after initially testing in mice, the cells were injected back into the bone marrow donor. These cells appeared to behave the same as regular red blood cells and so the technique appears promising, although the challenge remains in scaling up the process to larger quantities.
Marine lugworm blood
Some animals can transport oxygen more effectively in their body than humans can. The marine lugworm, Arenicola marina is a champion oxygen-hugger. Indeed, the worms breathe through gills, like fish, but they spend half their lives out of water where they can survive for 6 hours without breathing. This is possible thanks to their special haemoglobin, the proteins in blood that carry oxygen, that is 40 times more oxygenating than its human counterpart. In human blood, one haemoglobin protein holds four oxygen molecules at a time whereas a lugworm haemoglobin protein can hold an amazing 156 O2 molecules, according to the researcher Franck Zal. And the more he looked into these worms, the more special the worms’ blood became.
As it turns out, the lugworm is a universal donor – it doesn’t carry any antigens on its surface that are responsible for the different blood types. This means that it can be used in humans and could change the face of medicine. In 2016, the first clinical trials tested the efficacy of the worm blood and showed that transplant organs stored in Hemo2Life worm blood allowed for a faster patient recovery and improved organ function compared to transplant organs stored in the traditional electrolyte preservation solution. In fact, the lugworm’s haemoglobin is so effective at transporting oxygen that organs immersed in their solution can survive for days without damage, rather than hours.
Since those tests, Hemo2Life has been used to safeguard a variety of organs, including lungs, pancreases, and a heart. It was even used in 2018 in France, for the second full face transplant and was instrumental in the procedure according to the surgeon, Pr Lantieri who stated “I will never do another transplant surgery without this product “.
Franck Zal’s company, Hemarina, is in the process of applying for certification to sell Hemo2Life throughout Europe and could provide enough Hemo2Life for all the organ transplants in France and still have more than one tonne of worm blood left over.
Zal has set his sights on other uses for lugworm blood such as worm blood bandages to treat chronic wounds, or a haemoglobin-based oxygen carrier that could be used to treat sickle cell anaemia and even a gel-like oxygen carrier as a promising therapeutic solution for the treatment of periodontitis.
TIMELINE OF BLOOD TRANSFUSION
|
1628 Harvey |
The circulation of the blood (about 40 species) |
|
1656 Wren |
Injection into vascular system (dog) |
|
1665 Lower |
Transfusion of blood between dogs |
|
1666 Denis |
Transfusion from lamb to man |
|
1821 Provost and Dumas |
Blood transfusion revived haemorrhaged animals |
|
1824 Blundell |
Must use blood of the same species for transfusion |
|
1828 Blundell |
Blood transfusion saved women dying from post partum haemorrhage |
|
1869 Crile |
Serum of animals causes human red cells to clump |
|
1902 Landsteiner |
ABO blood group system |
|
1902 Carrel |
Technique of anastamosis of blood vessels (cat and dog) |
|
1915 Hustin and Lewisohn |
Citrate safely used as an anticoagulant (dog and rabbit) |
|
1916 Rous and Turner |
With pre-treatment, blood stored for two weeks (rabbits) |
|
1917 Robertson |
Institution of blood banks for wounded soldiers |
|
1933 |
First large blood bank established in Moscow |
References
References from previous version of this page. Current page has references hyper-linked.
- http://archive.thedailystar.net/newDesign/news-details.php?nid=238160
- Noha Barsoum & Charles Kleeman (2002) Now and Then, the History of Parenteral Fluid Administration Am J Nephrol 22:284–289
- Noha Barsoum & Charles Kleeman (2002) Now and Then, the History of Parenteral Fluid Administration Am J Nephrol 22:284–289
- Noha Barsoum & Charles Kleeman (2002) Now and Then, the History of Parenteral Fluid Administration Am J Nephrol 22:284–289
- Crile G (1907) Ann Surg 46, 329
- Hustin A (1914) J Med Brux 2, 436
- Lewisohn R (1915) Surg Gyn Obstet 21, 37
- Weil R (1915) JAMA 64, 425
- Rous P & Turner J (1916) J Exp Med 23, 219
- http://www.dailytelegraph.com.au/news/nsw/tamara-coakleys-life-saved-by-cows-blood/story-e6freuzi-1226050105216
- http://www.boston.com/business/healthcare/articles/2009/04/04/navy_rips_fda_for_blocking_clinical_trial/
- http://www.boston.com/business/healthcare/articles/2009/04/04/navy_rips_fda_for_blocking_clinical_trial/
- http://www.nature.com/news/2008/081111/full/news.2008.1219.html
- http://www.boston.com/business/healthcare/articles/2009/04/04/navy_rips_fda_for_blocking_clinical_trial/
- Hiroyama T, Miharada K, Sudo K, Danjo I, Aoki N, et al. (2008) Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS One 3: e1544
- Ryo Kurita et al. (2013) Establishment of Immortalized Human Erythroid Progenitor Cell Lines Able to Produce Enucleated Red Blood Cells PLoS One 8(3): e59890
- http://www.independent.co.uk/news/science/british-scientists-to-create-synthetic-blood-1651715.html
- http://www.news-medical.net/news/20130531/Novel-approach-to-create-unlimited-number-of-human-red-blood-cells-platelets-in-vitro.aspx
- http://www.scotsman.com/the-scotsman/health/scots-scientists-to-trial-synthetic-human-blood-1-2948081
- Giarrantana MC et al. (2011) Proof of principle for transfusion of in vitro–generated red blood cells Blood 118:19 5071-5079 doi: 10.1182/blood-2011-06-362038
Last edited: 13 June 2023 15:33
